Stealthy leprosy pathogen evades critical vitamin D-dependent immune response
UCLA findings point to new treatment pathways for infectious diseases
Advertisement
A team of UCLA scientists has found that the pathogen that causes leprosy has a remarkable ability to avoid the human immune system by inhibiting the antimicrobial responses important to our defenses.
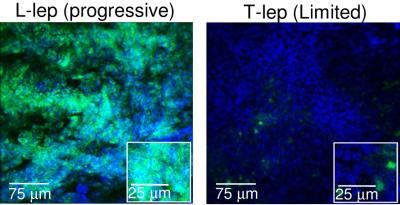
Skin biopsy sections from L-lep (progressive disease) and T-lep (limited disease) show the micro RNA hsa-mir-21 identified through a green fluorescent stain and skin nuclei identified with a blue fluorescent stain. Note more occurrence of hsa-mir-21 in progressive versus limited disease.
UCLA
In one of the first laboratory studies of its kind, researchers discovered that the leprosy pathogen Mycobacterium leprae was able to reduce and evade immune activity that is dependent on vitamin D, a natural hormone that plays an essential role in the body's fight against infections.
The pathogen manipulated micro-RNAs, tiny molecules made of ribonucleic acids that carry information and that help regulate genes to direct cell activity, including immune system defenses. Micro-RNAs are short RNAs that do not code information for proteins, which carry out all cell activity; rather, they bind to the RNAs that do code for proteins and block them.
Published in Nature Medicine, the findings demonstrate how an infectious disease pathogen like M. leprae can use micro-RNAs to impact the immune system's fight response.
"We may find that these tiny micro-RNAs can be exploited by pathogens to weaken our immune response," said the study's first author, Dr. Philip T. Liu, an assistant professor of medicine at the Orthopaedic Hospital Research Center and in the department of dermatology at the David Geffen School of Medicine at UCLA. "By better understanding how pathogens can escape our immune cells, we can design more effective therapies to boost our immune responses to these difficult to treat infections like leprosy."
For the study, researchers compared the micro-RNAs in human skin lesions from two types of leprosy: tuberloid leprosy, a milder infection that is more easily contained, and lepromatous leprosy, which is more serious and causes widespread infection throughout the body.
In the lab, the scientists identified 13 micro-RNAs that differed between the two types of leprosy. The micro-RNAs that were found to be more common in lepromatous leprosy seemed to target the genes important for directing key immune system cells, including macrophages and T cells.
The team found that a particular micro-RNA, hsa-mir-21, inhibited the gene activity of the vitamin D–dependent immune pathway used to help fight infection. When researchers neutralized the activity of hsa-mir-21 in macrophages, the cells were able to kill the bacteria again.
"The leprosy pathogen was able to effectively evade the host's immune response by regulating critical immune system genes," said senior investigator Dr. Robert Modlin, UCLA's Klein Professor of Dermatology and chief of dermatology at the Geffen School of Medicine. "It's like having the enemy sending a decoy message to your combat troops and telling them to lower their weapons."
To test the significance of this micro-RNA with other infectious diseases, the researchers also introduced hsa-mir-21 to human macrophages that were then infected with tuberculosis in the lab. Researchers found that the micro-RNA similarly blocked the ability of the macrophages to kill the bacteria.
Researchers also demonstrated that immune activation of the leprosy-infected immune cells decreased the leprosy bacteria's viability four-fold — but only when hsa-mir-21 activity was silenced. In fact, an over-expression of this micro-RNA blocked immune activity, resulting in a five-fold increase in bacterial viability.
"We were surprised at the devastating effects that even a single micro-RNA had on the ability of immune cells to fight the infections," Liu said.
In addition, the team showed that this micro-RNA was found in human immune cells only 18 hours after the onset of leprosy infection. The presence of the micro-RNA so early in the infection suggests it might play a role in actual disease development, the researchers said.
Further investigation of this single micro-RNA in leprosy may provide a framework for analyzing other micro-RNAs to help determine their cumulative role in regulating the immune response.
The micro-RNAs are small, and therefore it is possible to develop treatments which neutralize them, the researchers said.
























































