Merck Receives European Approval to Expand Use of Rebif in Patients with Early Multiple Sclerosis
Merck KGaA announced today that that the European Commission (EC) has approved extension of the indication of Rebif® (interferon beta-1a), Merck’s leading treatment for relapsing forms of multiple sclerosis (MS). This EC approval is for the use of Rebif 44 micrograms three times weekly in patients who have experienced a single demyelinating event, an early sign of the disease, and who are at high risk of converting to MS. This approval was based on the results of the REFLEX study, which showed the safety and efficacy of Rebif in this patient population.
“We are delighted by the European Commission decision,” said Dr Annalisa Jenkins, Head of Global Drug Development and Medical at the Merck Serono division. “Multiple sclerosis has an initial stage when clinical manifestations are not pronounced but irreversible neurological damage is taking place. Throughout the European Union, neurologists will now be able to prescribe Rebif for patients with early signs of this devastating disease.”
The new labelling for Rebif is valid immediately in all 27 member states of the
European Union.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Changes to Executive Board of Carl Zeiss AG - Dr. Ludwin Monz will be stepping down from his position as President and CEO of the Executive Board of Carl Zeiss Meditec AG at his own request
Sequenom Announces Exclusive Licensing Agreement with Genomic Nanosystems for Digital PCR Technologies and Methods Patents
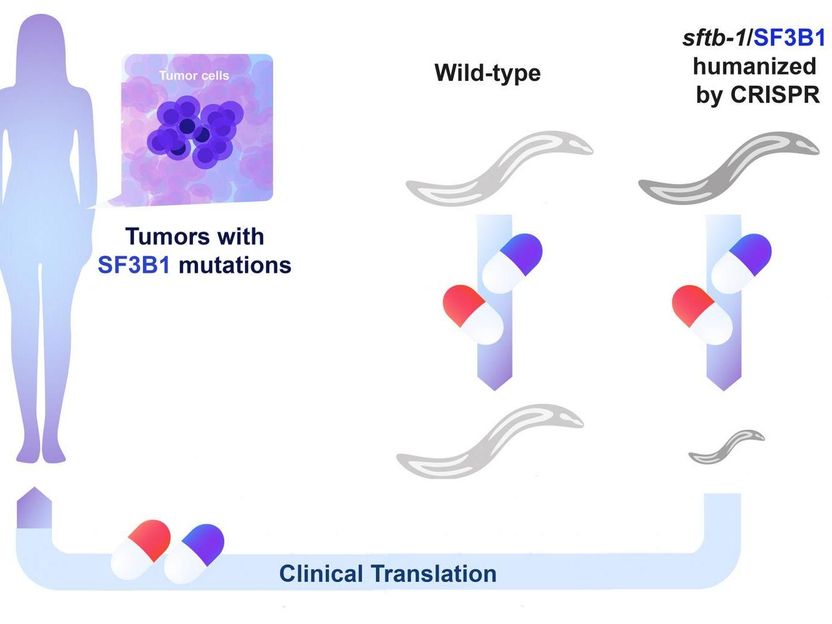
CRISPR-edited C. elegans identifies vulnerabilities in cancer
Stephanie_Schwabe
Enzyme_inhibitor
Biotechnology for soaking and liming