New study sheds light on evolutionary origin of oxygen-based cellular respiration
Advertisement
Researchers at the RIKEN SPring-8 Center in Harima, Japan have clarified the crystal structure of quinol dependent nitric oxide reductase (qNOR), a bacterial enzyme that offers clues on the origins of our earliest oxygen-breathing ancestors. In addition to their importance to fundamental science, the findings provide key insights into the production of nitrogen oxide, an ozone-depleting and greenhouse gas hundreds of times more potent than carbon dioxide.
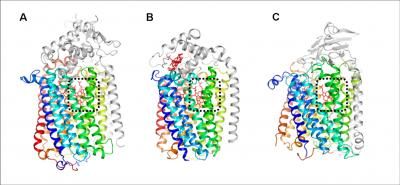
This graphic shows a comparison of the overall structures of the respiration enzymes. (A) qNOR, (B) cNOR, and (C) COX. These enzymes share similar structure of the core region shown with rainbow color. Each catalytic site denoted by black dotted line contains heme molecule shown as a red stick.
RIKEN
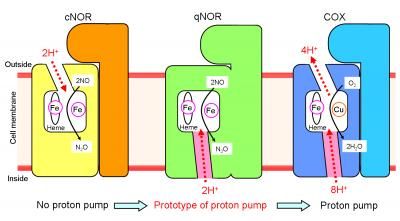
This is a schematic representation of the proton transfer pathways for the NO reduction reaction in cNOR and qNOR, and the proton pumping pathway in COX. The direction of the proton transfer is denoted by red dotted arrows. cNOR has a proton transfer pathway from the outside of the cell to the catalytic site for the NO reduction reaction. In sharp contrast to this, there is no proton transfer pathway from the outside of the cell in qNOR. Unexpectedly, however, we identified a proton transfer pathway from the inside of the cell to the catalytic site in qNOR. In COX, protons are pumped from the inside to the outside of the cell through the catalytic site. The location of the proton transfer pathway we identified in qNOR (pink color) is similar to a part of the proton pumping pathway in COX.
RIKEN
As the central process by which cells capture and store the chemical energy they need to survive, cellular respiration is essential to all life on this planet. While most of us are familiar with one form of respiration, whereby oxygen is used to transform nutrients into molecules of adenosine triphosphate (ATP) for use as energy ("aerobic respiration"), many of the world's organisms breathe in a different way. At the bottom of the ocean and in other places with no oxygen, organisms get their energy instead using substances such as nitrate or sulfur to synthesize ATP, much the way organisms did many billions of years ago ("anaerobic respiration").
While less well-known, this latter type of cellular respiration is no less important, fuelling the production of most of the world's nitrous oxide (N2O), an ozone depleting and greenhouse gas 310 times more potent than carbon dioxide. As the enzyme responsible for catalyzing the reactions underlying anaerobic respiration, nitric oxide reductase (NOR) has attracted increasing attention in environmental circles. The mystery of NOR's catalyzing mechanism, however – which accounts for a staggering 70% of the planet's N2O production – remains largely unsolved.
With their latest research, the team sought an answer to this mystery in the origin of an evolutionary innovation known as the "proton pump". To accelerate ATP-synthesis, aerobic organisms harness the potential of an electrochemical concentration gradient across the cell, created by "pumping" protons out using energy from an oxygen reduction reaction. The enzyme powering this mechanism, cytochrome oxidase (COX), is genetically and structurally similar to NOR, suggesting a common ancestor. No evidence of any "pump", however, has been detected in anaerobic organisms.
That is, until now. Using radiation from the RIKEN SPring-8 facility in Harima, Japan, the world's largest synchrotron radiation facility, the researchers probed the 3D structure of qNOR and discovered a channel acting as a proton transfer pathway for a key catalytic reaction. While not itself a proton pump, the position and function of this pathway suggest it is an ancestor of the proton pump found in COX. The finding thus establishes first-ever evidence for a proton pump in anaerobic organisms, shedding light onto the mysterious mechanisms governing the production of nitrogen oxide and the evolutionary path that led to their emergence.


























































