MHRA Issues GMP Certification to AMRI UK Facility
AMRI announced that the Medicines and Healthcare Regulatory Agency (MHRA) of the UK government has issued a Good Manufacturing Practice (GMP) certificate for AMRI's manufacturing facility in Holywell, Wales, UK, following an inspection in October 2011. The MHRA inspection follows a U.S. Food and Drug Administration audit of the facility in June 2011, in which no formal observations were issued. The successful completion of the US FDA and UK MHRA inspections means that AMRI's Holywell facility can now produce registered intermediates and active ingredients for use in humans and expands the range of projects that AMRI can now conduct at this facility.
AMRI Vice President, Pharmaceutical Development and Manufacturing Steven R. Hagen, Ph.D. said, "This certification completes an excellent year of regulatory cGMP audits for the Holywell facility and places it in a prime position to attract new business. The certification also closes a highly successful year of numerous audits across our global facilities that align us with best industry practices."
In 2011, AMRI's Rensselaer facility, which provides large-scale cGMP manufacturing, was inspected by FDA, in which no formal observations were issued. In addition, the Company received an approval in February 2011 from the Italian Medicines Agency (AIFA) for its Burlington, Mass. facility to manufacture a commercial drug product for a customer in the European Union.
Most read news
Organizations
Other news from the department politics & laws

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Neurobiology: Washing machine for the brain
Category:Jewish_medical_organizations

Mutating Hepatitis Viruses Make Drug Treatment More Difficult - Study paves the way for the next generation of active substances
Category:Branched-chain_amino_acids
Amanita_velosa
Diagnosis_of_Asperger_syndrome
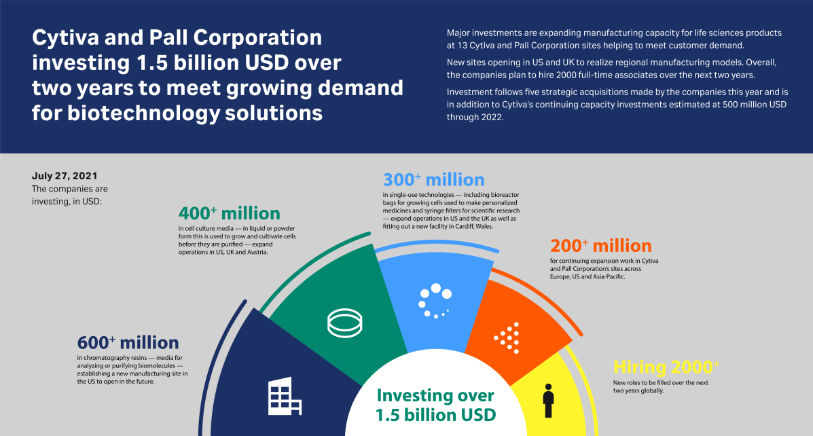
Cytiva and Pall Corporation investing 1.5 billion USD over two years to meet growing demand for biotechnology solutions - Major investments are expanding manufacturing capacity for life sciences products at 13 Cytiva and Pall Corporation sites helping to meet customer demand.
Experimental antibiotic treats deadly MRSA infection

A more effective hydrogel for healing wounds
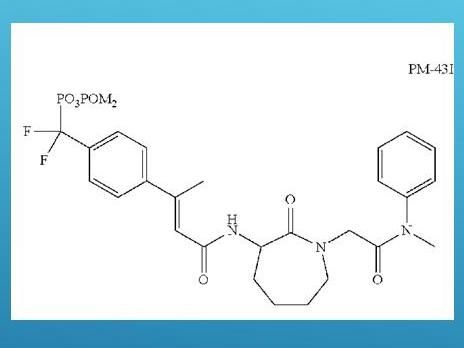
David vs Goliath: How a small molecule can defeat asthma attacks
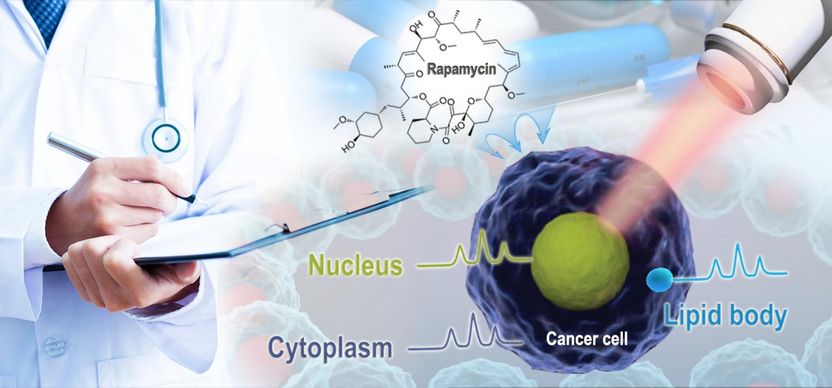
Single-cell test can reveal precisely how drugs kill cancer cells - New method sees how pharmaceuticals induce cancer cell death and how cancer cells adapt
















































