Novartis to restructure US business
US General Medicines restructuring results in reduction of 1,960 positions
Novartis Pharmaceuticals announced that the company plans to strengthen its long-term competitive position in anticipation of the Diovan® (valsartan) patent expiration and an expected reduction in demand for Rasilez®/Tekturna® (aliskiren) following termination of the ALTITUDE clinical study. The company will reduce its cost base with the current restructuring focused on the US market.
"We recognize that the next two years will be challenging in the Pharmaceuticals Division and we are proactively making these changes to further focus our pipeline on the best opportunities and align our market position on our growth brands," said David Epstein, Division Head of Novartis Pharmaceuticals. "These are difficult but necessary decisions that will free up resources to invest in the future of our business which we view as well suited to bring new valuable therapies to patients and payors."
A central element of the plan is a restructuring of the General Medicines business in the important US market, where Novartis Pharmaceuticals will continue to focus on expanding its presence in specialty businesses aligned with the product portfolio and pipeline. As a result, the field force is planned to be reduced by approximately 1,630 positions and headquarters functions will realign to support the new organization, resulting in an additional reduction of approximately 330 positions. The changes are planned to take effect in the second quarter of 2012, and associates will be notified in early April, 2012. All reductions will be handled in a manner consistent with the Novartis commitment to fair and respectful treatment of associates. Outplacement and other support services will be available to impacted associates as well as redeployment opportunities, where they exist, within the Novartis Group of companies.
The restructuring was prepared to respond to the loss of patent exclusivity for Diovan, the market-leading hypertension medication, expected in the US in September 2012. The plan has been accelerated after the ALTITUDE study was halted following the recommendation from the Data Monitoring Committee overseeing the trial. The study was investigating Rasilez/Tekturna in a high-risk population of patients with type-2 diabetes and renal impairment. As a precautionary measure Novartis Pharmaceuticals ceased all promotion of Rasilez/Tekturna-based products for use in combination with an angiotensin converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB). Novartis Pharmaceuticals, in consultation with health authorities, is now recommending that hypertensive patients with diabetes should not be treated with Rasilez/Tekturna in combination with an ACE-inhibitor or ARB. Patient safety is the highest priority for Novartis and we are in continuing dialogue with health authorities worldwide to establish the most appropriate next steps.
The restructuring is expected to result in an exceptional charge of approximately USD 160 million to be recognized in the results for the first quarter of 2012. It is planned to produce full-year savings of approximately USD 450 million as of 2013, about half of which is expected to be realized in 2012 due to reorganization timelines. Together with ongoing productivity programs, the company plans to continue to ensure that the product portfolio and research pipeline are fully invested in to sustain growth.
A reassessment of the future sales potential of Rasilez/Tekturna in light of the ALTITUDE results has led to an exceptional charge of approximately USD 900 million (of which approximately USD 800 million are non cash) to be recognized in the fourth quarter of 2011. The charge comprises impairments to intangible and manufacturing assets and excess inventory together with trial wind down and other exit costs. The accounting charge is triggered by lower sales expectations and does not seek to anticipate the results of our ongoing discussions with health authorities concerning Rasilez/Tekturna.
In addition, Novartis Pharmaceuticals will recognize an exceptional charge of approximately USD 160 million in the fourth quarter of 2011 related to termination of the PRT128 (elinogrel) and SMC021 (oral calcitonin) programs.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
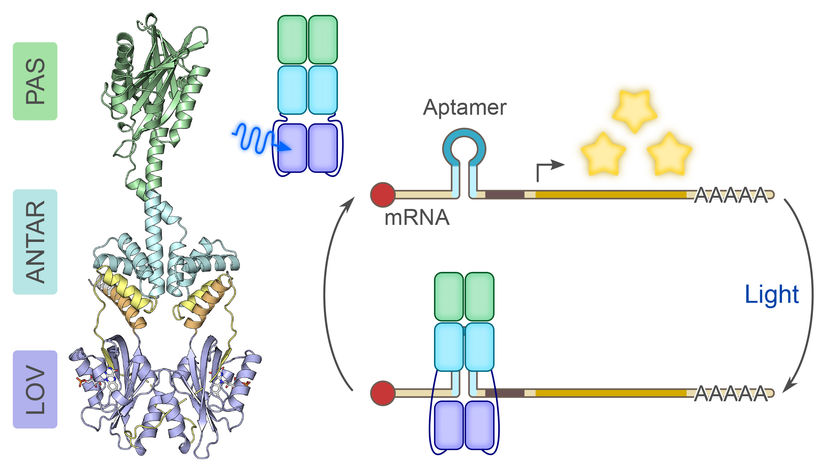
Blue light for RNA control - Researchers regulate the activity of RNA molecules
New discovery: Nanometric imprinting on fiber
Ecology insights improve plant biomass degradation by microorganisms

Halozyme withdraws proposal to acquire Evotec - Unwillingness to engage in discussion

Artificial intelligence predicts the future of artificial intelligence research - AI algorithm predicts the direction in which your research field is likely to evolve
Birch bark compounds offer a wealth of business opportunities

ACHEMA Start-up Award: Ten start-ups that want to change the process industry - The finalists are nominated

PreOmics announces early investors’ exit and closing of Series B financing - Bruker is now a majority investor in PreOmics
More than skin deep

Algae as living biocatalysts for a green industry - Discovery could lead to more environmental protection in the chemical industry