Novartis study showed ACZ885 provided substantial symptom relief in 84% of patients with the most serious form of childhood arthritis
Second pivotal Phase III trial ongoing; worldwide regulatory submissions planned for 2012
Novartis announced positive results of the first pivotal Phase III trial of ACZ885 (canakinumab) in patients with systemic juvenile idiopathic arthritis (SJIA), a rare and serious childhood auto-inflammatory disease. The results, presented at the 2011 European Pediatric Rheumatology Congress in Bruges, Belgium, showed all primary and secondary endpoints of the study were met.
Most ACZ885 patients (83.7%) experienced at least a 30% improvement in symptoms vs. 9.8% for placebo (p<0.0001) and a third of ACZ885 patients (32.6%) achieved a 100% improvement vs. 0% for placebo (p=0.0001)[2]. ACZ885 is an investigational, fully human monoclonal antibody that neutralizes interleukin-1 beta (IL-1 beta), which is a key driver of inflammation in SJIA.
"These data suggest that ACZ885 could become an important treatment option for children living with SJIA, the most difficult-to-treat and severe form of juvenile arthritis, potentially transforming their lives," said Professor Pierre Quartier, MD, one of the study investigators and Pediatric Rheumatologist Pediatric Immuno-Haematology and Rheumatology Unit, Necker-Enfants Malades Hospital, Paris, France. "ACZ885 provided rapid and long-lasting symptom relief by targeting interleukin-1 beta, a key inflammatory mediator of the disease."
"These results are a positive development for patients suffering from this very severe auto-inflammatory condition," said David Epstein, Head of the Pharmaceuticals Division of Novartis. "We are committed to investigating ACZ885 in a range of inflammatory diseases where interleukin-1 beta plays a key role and high unmet medical needs exist."
Therapies traditionally used to treat SJIA can only partially mitigate symptoms and do not prevent the long-term damage of the disease. Long-term steroid use designed to treat SJIA symptoms can also contribute to slowed growth and delayed puberty.
The results of a second pivotal Phase III trial, aimed at determining whether ACZ885 can extend the time to next flare and reduce or eliminate corticosteroid use, will be presented later this year. Worldwide regulatory submissions for ACZ885 in SJIA are planned for 2012.
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
Organ bioprinting gets a breath of fresh air - Bioengineers clear major hurdle on path to 3D printing replacement organs
Eiger BioPharmaceuticals Acquires Exclusive License to Novel Hepatitis C Virus (HCV) Technology From Stanford University

In neurodegenerative diseases, brain immune cells have a “ravenous appetite” for sugar - The interpretation of certain brain scans needs rethinking
Low levels of natural antibodies behind stroke
Cell Medica strengthens team with key appointment to senior management
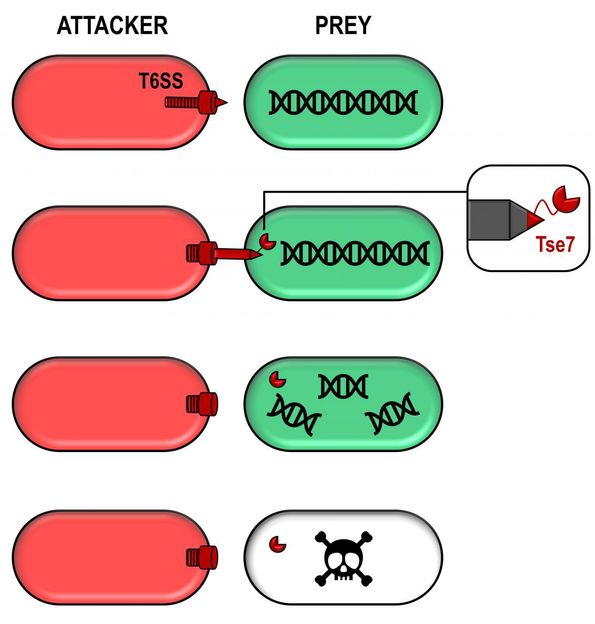
A toxic bullet involved in bacterial competition
'Origami' is reshaping DNA's future

Klara – A transparent fish for research on aging - Inactivation of pigmentation using CRISPR/Cas9
The putative skull of St. Bridget can be questioned
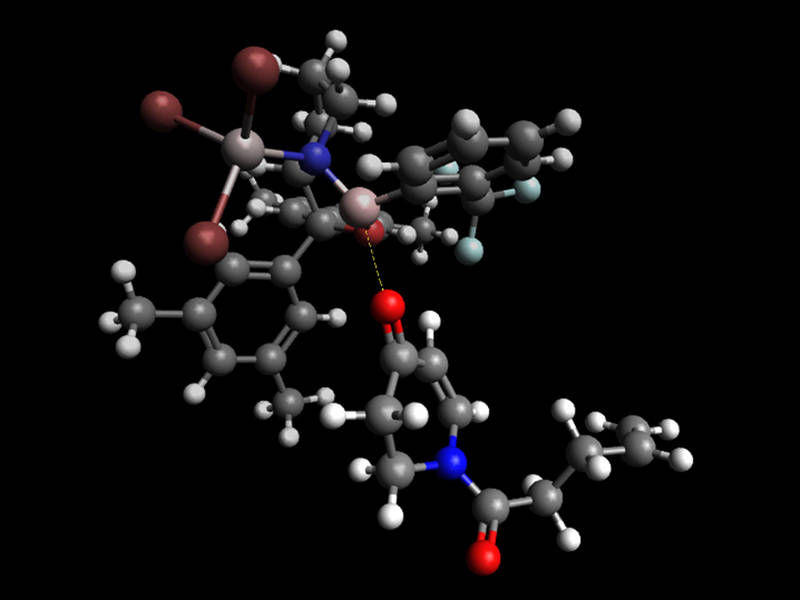
Targeted synthesis of natural products with light - Potential pathway for drug development using photoreactions