UF medicinal chemists modify sea bacteria byproduct for use as potential cancer drug
University of Florida researchers have modified a toxic chemical produced by tiny marine microbes and successfully deployed it against laboratory models of colon cancer.
Writing in ACS Medicinal Chemistry Letters, UF medicinal chemists describe how they took a generally lethal byproduct of marine cyanobacteria and made it more specifically toxic — to cancer cells.
When the scientists gave low doses of the compound to mice with a form of colon cancer, they found that it inhibited tumor growth without the overall poisonous effect of the natural product. Even at relatively high doses, the agent was effective and safe.
“Sometimes nature needs a helping human hand to further optimize these products of evolution to treat human diseases,” said Hendrik Luesch, Ph.D., an associate professor of medicinal chemistry at UF’s College of Pharmacy. “Based on what we learned about apratoxins’ mechanism of action, we knew this compound class had great potential for use in anticancer therapies; however, the natural product itself is too toxic to become a therapeutic.”
The researchers synthesized several apratoxin compounds that were similar to the original except for slight differences in composition, designing one that proved to be extremely potent against the cancer cells in cultures and in mice, but without the overwhelming toxicity.
The compound acts as a single agent to reduce levels of two types of proteins that are targeted by cancer research labs around the world — growth factors, and enzymes called tyrosine kinases, which act as receptors for the growth factors.
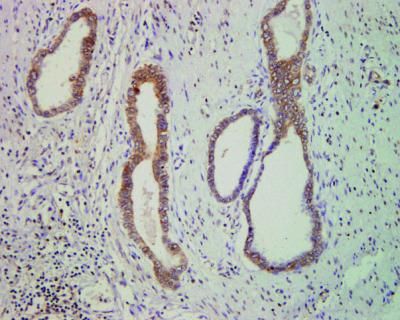
Known as apratoxin S4, the compound strips colon cancer cells of their ability to both secrete and use naturally occurring factors that fuel growth — something that Luesch, postdoctoral chemist Qi-Yin Chen, Ph.D., and assistant scientist Yanxia Liu, Ph.D., say is a powerful “one-two punch” against mushrooming populations of cancer cells.
The trio describes apratoxin’s dual action for the first time in today’s online publication, although Luesch presented early findings in May at the New York Academy of Sciences.
“This is an extremely interesting discovery that may have the potential to lead to a novel drug, but an extraordinary amount of additional research is needed before we will know. We can hope,” said David J. Newman, D.Phil., chief of the National Cancer Institute’s Natural Products Branch, who was not involved in the research. “Luesch has found a novel compound and a novel mechanism of action that stops the secretion of the receptor and the growth factor — as far as I am aware, this mechanism has only been shown in apratoxin at this time. If nothing else, he has shown us a new way to kill tumor cells and has revealed a new chemistry, and those are important steps.”
Apratoxin is produced by cyanobacteria, microbes that have evolved toxins to fend off predators and cope with harsh conditions in a marine environment. Collectively known as blue-green algae — a misnomer because the single-celled organisms are not algae or members of the plant kingdom — a wide variety of cyanobacteria species exists in both sea and freshwater environments.
Like plants, cyanobacteria convert sunlight into energy through a process known as photosynthesis. But where plants exclusively use a green pigment called chlorophyll to capture light to make food, cyanobacteria also use a bluish pigment called phycocyanin.
In addition, cyanobacteria have the unique ability to use respiration as well as photosynthesis to acquire energy, making these organisms tiny chemical factories capable of producing many as-yet unidentified molecules that may be useful for health applications.
“Marine cyanobacteria produce a huge diversity of compounds,” said Luesch, who is also a member of the UF Shands Cancer Center. “About half of anticancer drugs are based on natural products. All but a couple of them are derived from terrestrial organisms, yet more than 70 percent of the Earth is covered by oceans, which presumably contain a number of therapeutic molecules with potentially novel biological activities. When we studied the biological effects of apratoxin, we predicted it would be particularly useful against colon cancer if we could engineer it to be more selective.”
Chen synthesized the apratoxins, while Liu carried out the biology and pharmacology experiments. More lab work is required before a drug based on apratoxin can be tested in patients with colon cancer, but Luesch believes apratoxin S4 is the first candidate to show the needed tumor selectivity, antitumor effects and potency to be effective. The UF Research Opportunity Fund and the Bankhead-Coley Cancer Research Program supported the study.