The 2011 Körber Prize goes to Stefan Hell
With a groundbreaking idea the physicist has overcome the diffraction resolution barrier in optical microscopy
Prof. Dr. Dr. h. c. Stefan Hell of the Max Planck Institute for Biophysical Chemistry in Göttingen is to receive the 2011 Körber European Science Prize endowed with 750,000 euros for his pioneering discoveries in the field of optics. Every year, the Körber Prize is awarded to an outstanding scientist working in Europe on particularly promising projects. The prizewinner is selected by an international trustee committee chaired by Prof. Dr. Peter Gruss, President of the Max Planck Society.
How deeply can we penetrate into the details of the visible world with optical microscopes? Previously, the law formulated by Ernst Abbe in 1873 was regarded as the absolute lower limit. Objects lying closer to each other than 200 millionths of a millimetre, i.e. about one two hundredth of a hair's breadth, can no longer be distinguished from one another. The reason for this is the wave nature of light, the half wavelength of which roughly corresponds to those 200 nanometres.
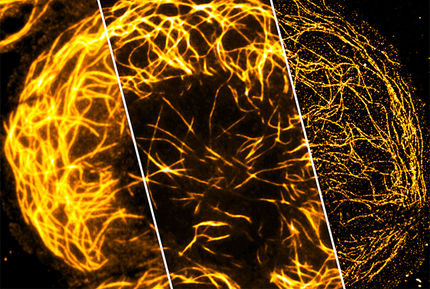
The STED (Stimulated Emission Depletion) microscopy, which the Göttingen-based physicist Stefan Hell invented and developed to application readiness, allows scientists to gain insights into the nano world far beyond this limit. Biologists and physiologists in particular value this breakthrough, because living cells or tissue can only be observed using optical microscopes. In 2008, for instance, neurophysiologists using the new resolution of only a few dozen nanometres succeeded in visualising the movements of tiny synaptic components for the first time. In addition, the concept underlying STED microscopy opened up new prospects for the further development of optical storage media.
Stefan Hell overcame Abbe's barrier in the imaging of fluorescent objects. In this process, which is used widely in biology and medical research, the specimens to be examined are marked with fluorescent molecules and illuminated – e.g. with a focussed laser beam. The beam excites the molecules so that they emit fluorescent light, thereby making the marked cell components visible. Here too, the fluorescent light emitted by the closely adjoining dots also becomes an indistinct blur, but Hell found a simple trick to break through Abbe's barrier. This ensures that the cell components illuminated by the excitation beam do not emit fluorescence simultaneously, but sequentially. To achieve this, Hell applies a second beam (STED beam) which temporarily prevents the fluorescent markers from emitting light, i.e. it switches them off.
In his STED microscope, this second, ring-shaped "switch-off" beam is superimposed with the approx. 200 nm circular focal area of the excitation beam, where it keeps all the specimen's features dark, except those at the very centre of the ring. Only the molecules in this zone are registered. Scanning the two beams across the specimen also separates the cell components which are much closer together than 200 nm. The images are consequently yielded with fundamentally improved resolution.
Stefan Hell will receive the prize money for his research project on new fluorescent dyes which can be switched on and off with much less light. This would further increase the attainable resolution. Moreover, potentially harmful effects of the light on the observed cells and tissue could be reduced, as the intensity of the laser radiation required would be lower.
Stefan Hell has been a Director at the Max Planck Institute for Biophysical Chemistry in Göttingen since 2002. Born in Banat, Romania in 1962, he studied physics at the University of Heidelberg, where he also did his PhD. Following research stations at the EMBL in Heidelberg and the universities of Turku and Oxford, in addition to his work in Göttingen he became a division head at the German Cancer Research Centre in Heidelberg.
Other news from the department science
These products might interest you
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.