Dynamics of crucial protein 'switch' revealed
Cell signaling networks tied to diabetes and cancer
Researchers at the University of Texas Medical Branch at Galveston and the University of California-San Diego School of Medicine have published a study that offers a new understanding of a protein critical to physiological processes involved in major diseases such as diabetes and cancer. This work could help scientists design drugs to battle these disorders. The article was published in the Journal of Biological Chemistry.
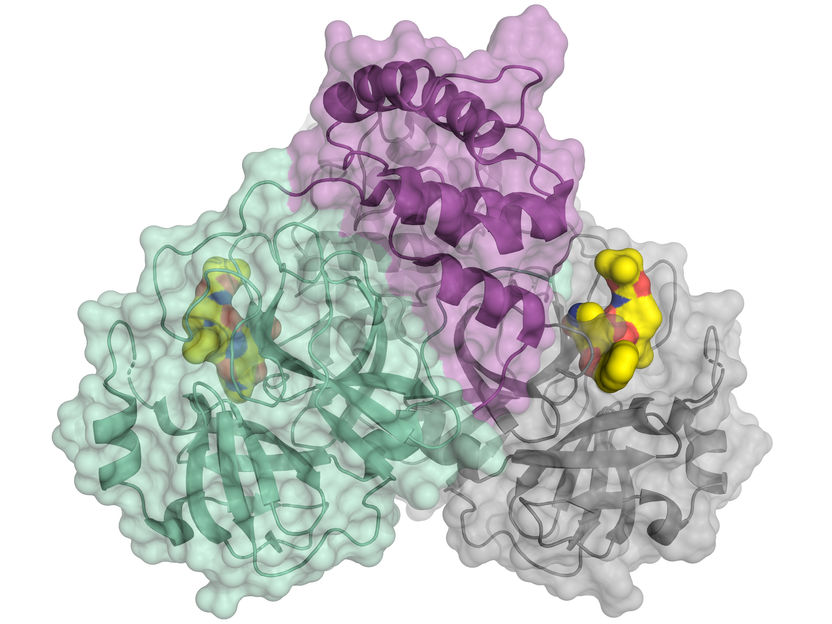
"This study applied a powerful protein structural analysis approach to investigate how a chemical signal called cAMP turns on one of its protein switches, Epac2," said principal investigator Xiaodong Cheng, professor in the Department of Pharmacology and Toxicology and member of the Sealy Center for Structural Biology and Molecular Biophysics at UTMB.
The cAMP molecule controls many physiological processes, ranging from learning and memory in the brain and contractility and relaxation in the heart to insulin secretion in the pancreas. cAMP exerts its action in cells by binding to and switching on specific receptor proteins, which, when activated by cAMP, turn on additional signaling pathways.
Errors in cell signaling are responsible for diseases such as diabetes, cancer and heart failure. Understanding cAMP-mediated cell signaling, in which Epac2 is a major player, likely will facilitate the development of new therapeutic strategies specifically targeting the cAMP-Epac2 signaling components, according to the researchers.
The project involved an ongoing collaboration between Cheng's research group at UTMB, experts in the study of cAMP signaling, and UCSD professor of medicine Virgil Woods Jr. and colleagues at UCSD, pioneers in the development and application of hydrogen/deuterium exchange mass spectrometry (DXMS) technology. Compared with other protein-analysis techniques, DXMS is especially good at studying the structural motion of proteins.
Using this novel approach, the investigators were able to reveal, in fine detail, that cAMP interacts with its two known binding sites on Epac2 in a sequential fashion and that binding of cAMP changes the shape of the protein in a very specific way – switching on its activity by exposing further signaling interaction sites on Epac2.
"DXMS analysis has proved to be an amazingly powerful approach, alone or in combination with other techniques, in figuring out how proteins work as molecular machines, changing their shapes – or morphing – in the normal course of their function," said Woods. "This will be of great use in the identification and development of therapeutic drugs that target these protein motions."
Most read news
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.