New DNA nanoforms take shape
Miniature architectural forms—some no larger than viruses—have been constructed through a revolutionary technique known as DNA origami. Now, Hao Yan, Yan Liu and their colleagues at Arizona State University's Biodesign Institute have expanded the capability of this method to construct arbitrary, two and three-dimensional shapes, mimicking those commonly found in nature.
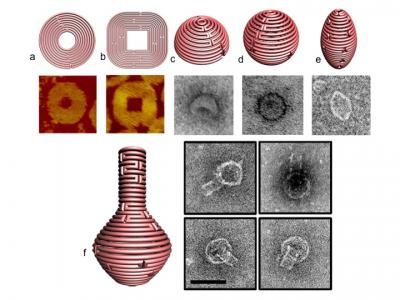
Figure 1 a and b display schematics for 2-D nanoforms with accompanying AFM images of the resulting structures. 1 c-e represent 3D structures of hemisphere, sphere and ellipsoid, respectively, while figure 1f shows a nanoflask, (each of the structures visualized with TEM imaging).
The Biodesign Institute Arizona State University
Such diminutive forms may ultimately find their way into a wide array of devices, from ultra-tiny computing components to nanomedical sentries used to target and destroy aberrant cells or deliver therapeutics at the cellular or even molecular level.
In today's issue of Science, the Yan group describes an approach that capitalizes on (and extends) the architectural potential of DNA. The new method is an important step in the direction of building nanoscale structures with complex curvature—a feat that has eluded conventional DNA origami methods. "We are interested in developing a strategy to reproduce nature's complex shapes," said Yan.
The technique of DNA origami was introduced in 2006 by computer scientist Paul W.K. Rothemund of Caltech. It relies on the self-assembling properties of DNA's four complementary base pairs, which fasten together the strands of the molecule's famous double-helix. When these nucleotides, labeled A, T, C, and G, interact, they join to one another according to a simple formula—A always pairs with T and C with G.
Nanodesigners like Yan treat the DNA molecule as a versatile construction material—one they hope to borrow from nature and adapt for new purposes. In traditional DNA origami, a two-dimensional shape is first conceptualized and drawn. This polygonal outline is then filled in using short segments of double-stranded DNA, arranged in parallel. These segments may be likened to pixels—digital elements used to create words and images displayed on a computer screen.
Indeed, Rothemund and others were able to use pixel-like segments of DNA to compose a variety of elegant 2-dimensional shapes, (stars, rhomboids, snowflake forms, smiley faces, simple words and even maps), as well as some rudimentary 3-dimensional structures. Each of these relies on the simple rules of self-assembly guiding nucleotide base paring.
Once the desired shape has been framed by a length of single-stranded DNA, short DNA "staple strands" integrate the structure and act as the glue to hold the desired shape together. The nucleotide sequence of the scaffold strand is composed in such a way that it runs through every helix in the design, like a serpentine thread knitting together a patchwork of fabric. Further reinforcement is provided by the staple strands, which are also pre-designed to attach to desired regions of the finished structure, through base pairing.
"To make curved objects requires moving beyond the approximation of curvature by rectangular pixels. People in the field are interested in this problem. For example, William Shih's group at Harvard Medical School recently used targeted insertion and deletion of base pairs in selected segments within a 3D building block to induce the desired curvature. Nevertheless, it remains a daunting task to engineer subtle curvatures on a 3D surface, " stated Yan.
"Our goal is to develop design principles that will allow researchers to model arbitrary 3D shapes with control over the degree of surface curvature. In an escape from a rigid lattice model, our versatile strategy begins by defining the desired surface features of a target object with the scaffold, followed by manipulation of DNA conformation and shaping of crossover networks to achieve the design," Liu said.
To achive this idea, Yan's graduate student Dongran Han began by making simple 2-dimensional concentric ring structures, each ring formed from a DNA double helix. The concentric rings are bound together by means of strategically placed crossover points. These are regions where one of the strands in a given double helix switches to an adjacent ring, bridging the gap between concentric helices. Such crossovers help maintain the structure of concentric rings, preventing the DNA from extending.
Varying the number of nucleotides between crossover points and the placement of crossovers allows the designer to combine sharp and rounded elements in a single 2D form, as may be seen in figure 1 a & b, (with accompanying images produced by atomic force microscopy, revealing the actual structures that formed through self-assembly). A variety of such 2D designs, including an opened 9-layer ring and a three-pointed star, were produced.
The network of crossover points can also be designed in such a way as to produce combinations of in-plane and out-of-plane curvature, allowing for the design of curved 3D nanostructures. While this method shows considerable versatility, the range of curvature is still limited for standard B form DNA, which will not tolerate large deviations from its preferred configuration—10.5 base pairs/turn. However, as Jeanette Nangreave, one of the paper's co-authors explains, "Hao recognized that if you could slightly over twist or under twist these helices, you could produce different bending angles."
Combining the method of concentric helices with such non-B-form DNA (with 9-12 base pairs/turn), enabled the group to produce sophisticated forms, including spheres, hemispheres, ellipsoid shells and finally—as a tour de force of nanodesign—a round-bottomed nanoflask, which appears unmistakably in a series of startling transmission electron microscopy images (see figure 1, c-f )
"This is a good example of teamwork in which each member brings their unique skills to the project to make things happen." The other authors include Suchetan Pal and Zhengtao Deng, who also made significant contributions in imaging the structures.
Yan hopes to further expand the range of nanoforms possible through the new technique. Eventually, this will require longer lengths of single-stranded DNA able to provide necessary scaffolding for larger, more elaborate structures. He credits his brilliant student (and the paper's first author) Dongran Han with a remarkable ability to conceptualize 2- and 3D nanoforms and to navigate the often-perplexing details of their design. Ultimately however, more sophisticated nanoarchitectures will require computer-aided design programs—an area the team is actively pursuing.
The successful construction of closed, 3D nanoforms like the sphere has opened the door to many exciting possibilities for the technology, particularly in the biomedical realm. Nanospheres could be introduced into living cells for example, releasing their contents under the influence of endonucleases or other digestive components. Another strategy might use such spheres as nanoreactors—sites where chemicals or functional groups could be brought together to accelerate reactions or carry out other chemical manipulations.