Cellmid completes Proof of Concept Study for Heart Attack
Animal study demonstrates midkine treatment reduces heart muscle death by 27% following heart attack
Cellmid Limited has completed its milestone preclinical studies into the efficacy of midkine (MK) for the treatment of acute myocardial infarction (AMI). Total dose of 0.18 mg/kg MK performed best and reduced the area of heart muscle damage by approximately 27% when compared to untreated animals undergoing the same procedure. These studies confirm Cellmid’s own earlier research findings that MK does indeed reduce heart damage due to ischemia and reperfusion injury.
The significant improvement demonstrated by the studies’ results mean that large animal trials can now commence. Further, manufacturing of GMP quality MK can also proceed in preparation for clinical trials.
The preclinical study was conducted by Cellmid’s collaborator, Pharmahungary, within their specialist cardiovascular research facilities. MK was given to rats intravenously in single doses and in follow up intravenous infusions. MK was also safe and well tolerated, with no difference in adverse events between MK treated and untreated controls.
“A reduction of around 27% in infarct size is very encouraging”, said CEO of Pharmahungary, Dr Peter Ferdinandy. “Pharmahungary’s study was extremely rigorous. Samples were blinded for analysis and the area of heart muscle death was measured using their state-of-the-art imaging software Infarctsize™” added Cellmid’s Head of Product Development, Darren Jones.
As well as conducting the pivotal MK efficacy study, Pharmahungary also evaluated MK in dose-ranging and acute safety/tolerability studies. Dose-ranging studies showed that MK was effective at significantly reducing heart muscle death with doses between 0.1mg/kg and 1.0 mg/kg. Furthermore, MK was safe and well tolerated at doses 16 times greater than the most effective dose of 0.18 mg/kg, with no cardiovascular toxicities observed.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
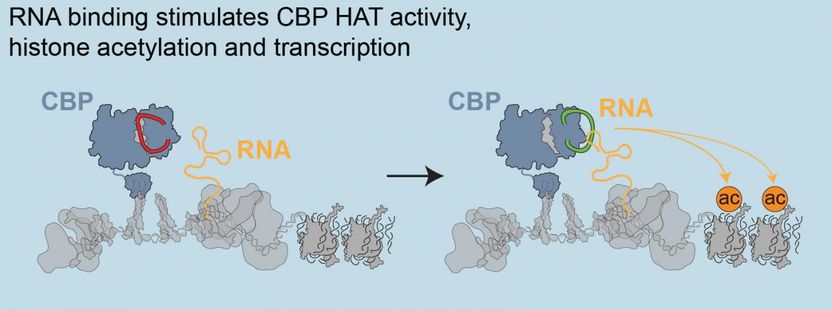
'Mysterious' non-protein-coding RNAs play important roles in gene expression
David_Kerns