Intercell announces start of pivotal Phase II/III study in India for vaccine to protect children from Japanese Encephalitis
Intercell AG and its partner Biological E. Ltd. announced the start of a pediatric Phase II/III study for the vaccine to protect children from Japanese encephalitis. The vaccine is manufactured in India by Biological E. and is based on Intercell's technology, which was successfully used to gain product licensure of the adult vaccine in Europe, the United States, Canada and Hong Kong (IXIARO®) as well as in Australia (JESPECT®).
This randomized and controlled study will be the first pivotal Phase II/III study in an endemic region towards licensure of the pediatric JE vaccine. The study will enroll healthy children aged one to below three years across multiple sites in India. This Phase II/III trial aims at investigating safety and immunogenicity compared to a licensed competitor Japanese Encephalitis vaccine.
"We are very pleased with the progress in our collaboration with Biological E. which undertakes the development of this vaccine to protect children living in endemic areas threatened by this terrible disease. The start of the current Phase II/III study is a major step towards making our modern cell culture-derived product available in Asia, and supports our global vision for our Japanese Encephalitis franchise", states Thomas Lingelbach, Chief Operating Officer of Intercell AG.
In 2005, Intercell and Biological E. signed a contract for the development, manufacturing, marketing and distribution in India and the Indian subcontinent of Intercell's Japanese Encephalitis vaccine. The vaccine's further regulatory approval route for other Asian territories is expected through the World Health Organization – Novartis will be responsible for the marketing and distribution in these regions.
Data from a previous Phase II study conducted in 2007 in India by Biological E. with vaccine manufactured at Intercell’s Scottish manufacturing site suggested that the vaccine has a comparable excellent immunogenicity and safety profile in young children (one to below three years of age) as in adults, even if only half of the adult dose is applied.
In 2010, Biological E. conducted a trial in 20 healthy adults, confirming the safety profile seen in that previous Phase II trial with product manufactured at Biological E.'s facility.
Following the approval and launch of Intercell's vaccine against Japanese Encephalitis for adult travelers and military personnel in Europe, the USA and Australia, the development of a vaccine to protect children in endemic areas from Japanese Encephalitis has been a major goal of the Company.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Quotient Clinical and Bend Research Announce Collaboration To Accelerate Advancement of New Medicines

Taking aim at skin bacteria - Enzyme treatment of skin samples improves microbiome analysis

SeaBeLife awarded over €1.5 million in i-Nov innovation competition - Funding for company’s SeaBeEYE project will be used to develop new therapeutic approach for geographic atrophy – advanced form of dry AMD
Autifony Therapeutics announces initiation of Phase IIa study for first-in-class drug to treat tinnitus
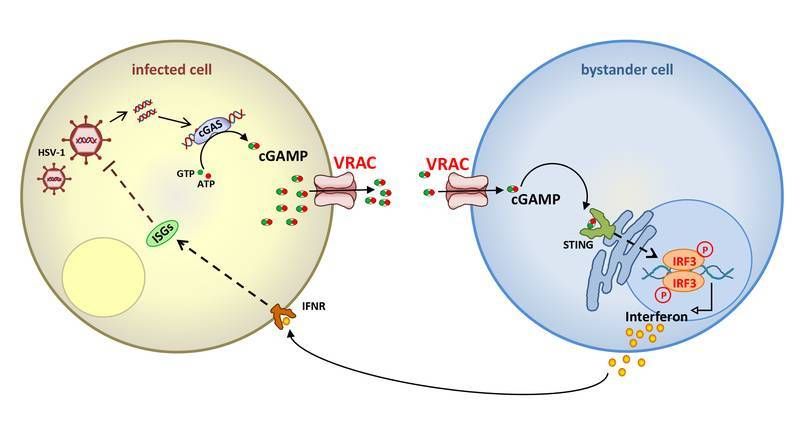
Ion channel VRAC enhances immune response against viruses - New strategies against DNA viruses and cancer
Selvita Signs a Drug Discovery Services Contract With Rottapharm Madaus
Novel gene drives development of different types of ovarian cancer
Discovery of 100 million-year-old regions of DNA shows short cut to crop science advances
Traditional_Chinese_medicine
Biogen Idec and Swedish Orphan Biovitrum Decide to Advance Long-Lasting Hemophilia A Therapy into a Registrational Trial
Sjögren-Larsson_syndrome