Medivir Announces Start of Phase 1a Trial of the Hepatitis C Polymerase Inhibitor TMC649128
Medivir AB announced the start of a phase 1a clinical trial with TMC649128 intended for the treatment of chronic hepatitis C virus infection. TMC649128 is a nucleoside NS5B polymerase inhibitor that has already demonstrated an attractive pre-clinical profile. It is anticipated that this profile would see TMC649128 be used in combination with HCV directly acting antiviral agents, given their high genetic barrier to resistance and antiviral activity across multiple HCV genotypes.
In pre-clinical studies, TMC649128 displayed in vitro activity across multiple HCV genotypes and a high genetic barrier to resistance.
The phase 1a trial is a double-blind, randomized, placebo-controlled single-ascending dose trial to assess the safety, tolerability and pharmacokinetics in healthy volunteers and will be conducted in Belgium. TMC649128 is being developed in collaboration with Tibotec Pharmaceuticals.
Medivir entered a Research and Development agreement in the field of hepatitis C virus polymerase with Ortho Biotech Products LP, an affiliate of Tibotec in May 2008. The development of TMC649128 falls under this agreement and by entering clinical development, a milestone payment of Euro 7 million has been triggered for payment to Medivir.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
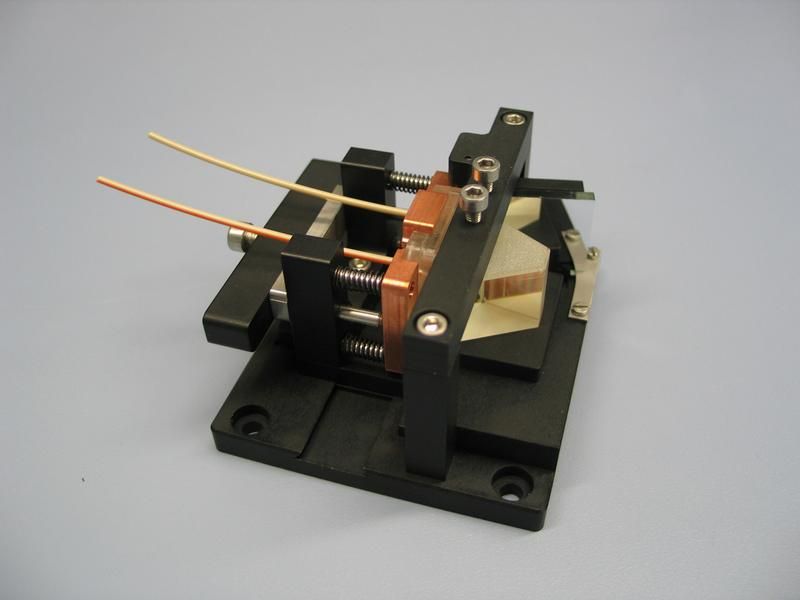
Blood diagnosis - chip-based and mobile

HACH LANGE AB - Sköndal, Sweden
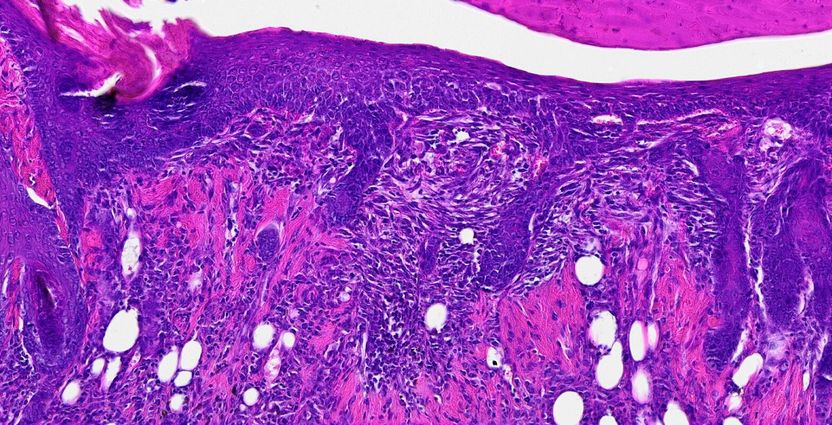
Less “Sticky” Cells Become More Cancerous - Researchers investigated mobility of cancer cells
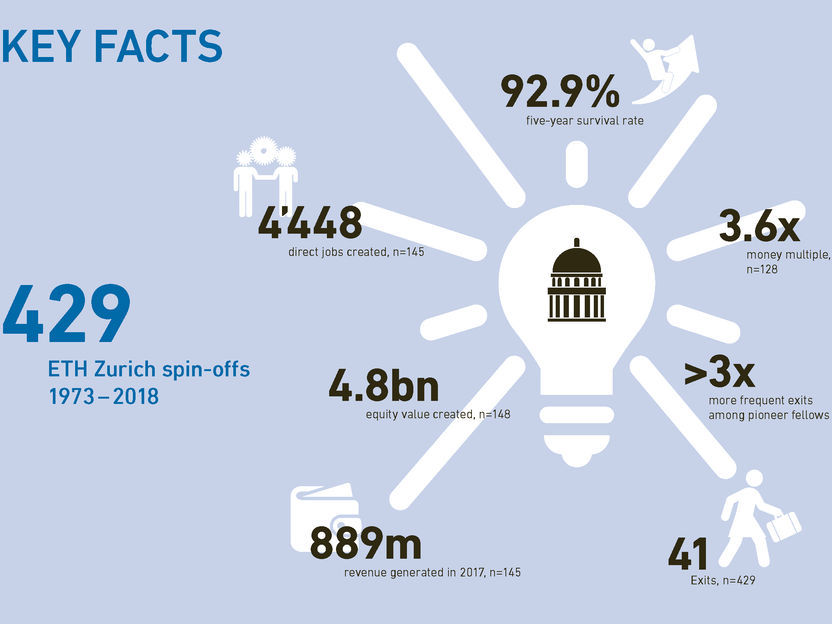
How ETH Zurich spin-offs strengthen the Swiss economy - ETH spin-offs are much more likely to make an exit than other Swiss start-ups
Epigenomics: US study suggests blood-based Septin9 CRC screening could save lives
New therapy to combat skin cancer - Neu-Ulm-based medical device producer and High-Tech Gründerfonds found spin-off Zimmer BioTech
Multidrug-resistant malaria spreading in Asia - Study reveals importance of genomic surveillance for malaria control strategies