FDA approves the Novartis quadrivalent meningococcal conjugate vaccine, Menveo, for use in children from 2 years of age
Novartis announced that it received approval from the US Food and Drug Administration (FDA) for the use of Menveo® for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y and W-135 in individuals 2 to 10 years of age. Menveo received initial FDA approval in 2010 for use in adolescents and adults from 11 to 55 years of age.
The FDA approval of Menveo for use in children 2 to 10 years of age is based on Phase III trial data involving 5,297 participants in that age group. In the pivotal trial, the safety and immunogenicity of Menveo against each of the four serogroups were compared with those of the other currently US-licensed ACW-135Y meningococcal conjugate vaccine. Novartis also agreed to conduct three post marketing studies.
Separately, Novartis received a Refuse To File (RTF) letter from the FDA regarding the Company's supplemental Biologics License Application (sBLA) for the use of Menveo in infants from 2 to 12 months. The sBLA had been submitted to the FDA in November 2010. Novartis plans to resubmit a sBLA in 2011 for the expanded use of Menveo in infants and toddlers from 2 months to 2 years old.
"The approval of Menveo for the use in children 2-10 years of age is another important step towards our goal to protect people of all ages against this devastating disease," said Andrin Oswald, Division Head of Novartis Vaccines and Diagnostics. "The response from the FDA on our Menveo infant file is disappointing. We believe that concerns raised are of procedural nature and plan to resubmit the sBLA within the next few months. This will also provide us with an opportunity to supplement the file with additional clinical data that support expanded use of Menveo in infants and toddlers from 2 months to 2 years old."
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents

Integrated DNA Technologies, BVBA - Löwen, Belgium
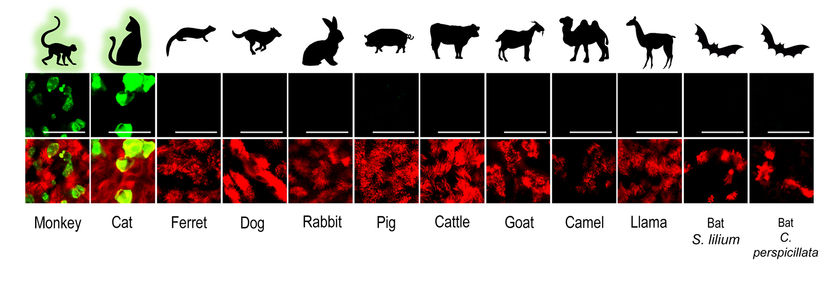
In vitro Zoo helps in understanding SARS-CoV-2 - Rhesus macaques and cats as potential spillback reservoirs for SARS-CoV-2

Analytik Jena AG with New Managing Director at French Subsidiary

Deep 6 AI - Pasadena, USA

New, portable tech sniffs out plant disease in the field

























































