Genzyme Successfully Meets First Milestones of FDA Consent Decree
All Filling and Finishing for U.S. Market Transferred Out of Allston Plant
Genzyme Corporation announced that the company has ended fill/finish operations within its Allston plant for products sold in the United States, as required by the FDA consent decree. All fill/finish activities for Cerezyme® (imiglucerase for injection), Myozyme® (alglucosidase alfa), Fabrazyme® (agalsidase beta) and Thyrogen® (thyrotropin alfa for injection) for the U.S. market now take place at Genzyme’s Waterford, Ireland plant, and at an external contract manufacturer. With this move, all previous restrictions on the marketing and distribution of Thyrogen within the United States have been lifted.
All remaining fill/finish activities in Allston for products sold outside of the United States must be ended by August 31, 2011. Genzyme is working closely with regulatory authorities globally to achieve this goal.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Epigenomics AG Receives Allowance for Key Technology Patent in Japan - Patent broadly covers HeavyMethyl, a core technology of Epigenomics' molecular diagnostic products
FORMAC strengthens management with appointment of CSO and COO
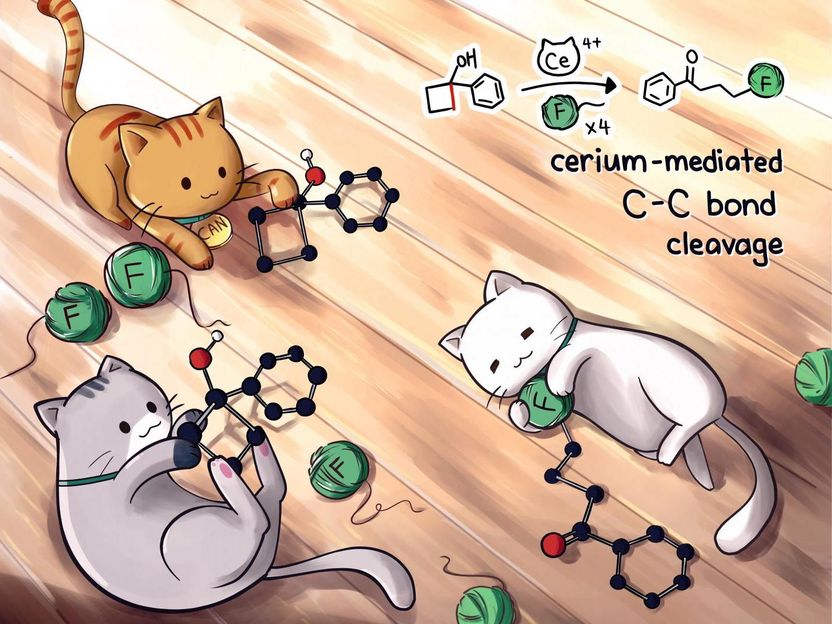
Cerium sidelines silver to make drug precursor - Rice University lab's process simplifies fluoroketone synthesis
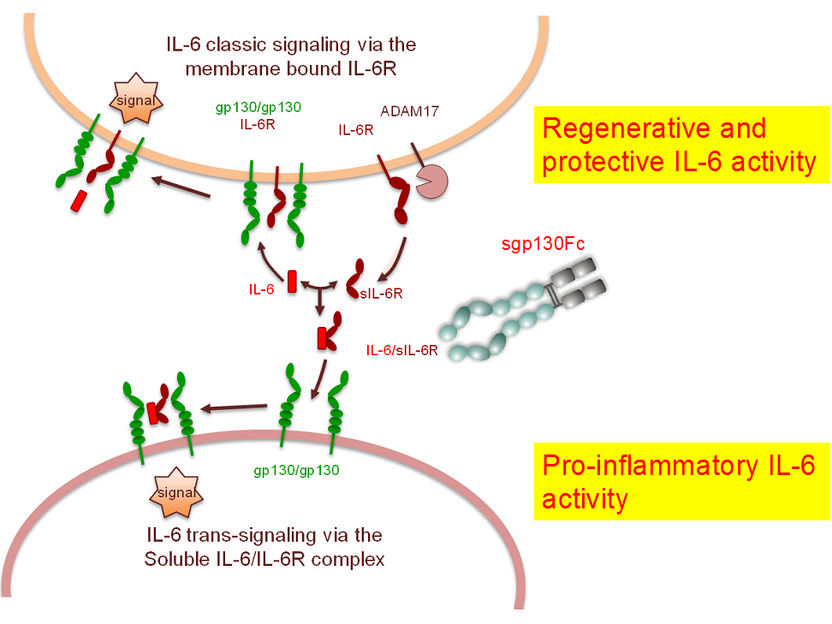
Anti-inflammatory principle also works against lung cancer and emphysema
Medical_students'_disease
-ase
Hirschsprung's_disease
Researchers find no difference in drugs for macular degeneration
Pickwickian_syndrome