Lundbeck and Merck sign exclusive commercialisation agreement for SYCREST (asenapine) sublingual tablets
Lundbeck expects to launch SYCREST in the EU at the beginning of 2011
H. Lundbeck A/S and Merck & Co., also known outside the United States and Canada as MSD, announced a commercialisation agreement for SYCREST® (asenapine) sublingual tablets (5 mg, 10 mg). Under the terms of the agreement, Lundbeck will pay an undisclosed fee as well as product supply payments in exchange for exclusive commercial rights to SYCREST® in all markets outside the United States, China and Japan. Lundbeck expects to launch SYCREST® in the European Union (EU), where it is already approved at the beginning of 2011. Merck will retain exclusive commercial rights to asenapine in the United States, China and Japan. Merck has launched asenapine in the United States under the brand name SAPHRIS® (asenapine) sublingual tablets (5 mg, 10 mg).
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
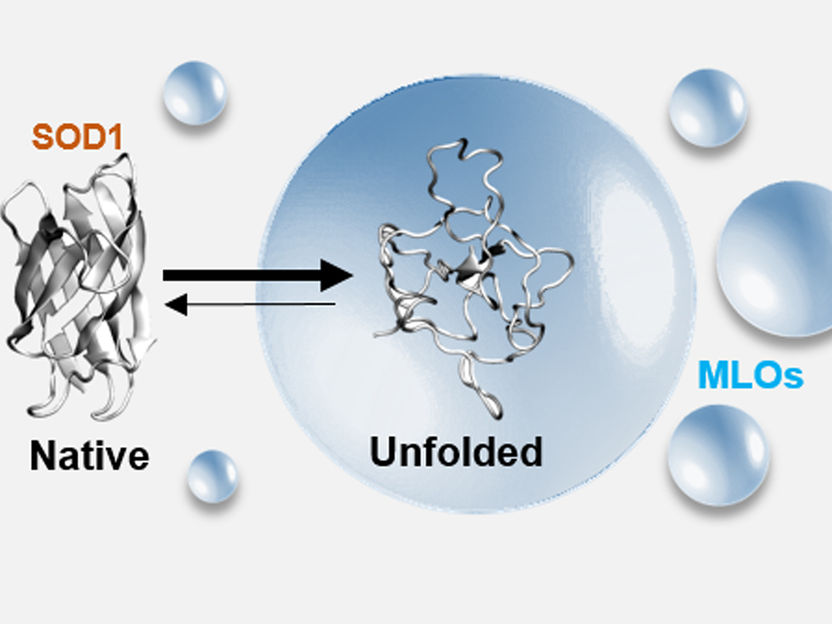
How proteins clump together - Molecular mechanisms in the aggregation of proteins in membraneless organelles
Provepharm signs license agreement with Daiichi Sankyo to provide methylthioninium chloride Proveblue solution for injection to Japan - Daiichi Sankyo will resolve the unmet need for an approved methylthioninium chloride injectable drug in Japan