AMT receives orphan drug designation from the U.S. Food and Drug Administration Duchenne Muscular Dystrophy gene therapy
Amsterdam Molecular Therapeutics (AMT) Holding N.V. announced that the U.S. Food and Drug Administration (FDA) has designated AMT-080, a gene therapy for Duchenne muscular dystrophy (DMD) as an orphan drug. In October 2009, the Committee for Orphan Medical Products of the European Medicines Agency granted AMT-080 orphan designation for the same indication in the European Union.
AMT has shown efficacy in studies of a preclinical model of DMD. These proof of concept studies demonstrated that AMT’s technology resulted in functional dystrophin synthesis in both the heart and skeletal muscles, leading to the prevention of muscular dystrophy. These data are strengthened by a study in which this gene therapy approach was shown to successfully restore dystrophin activity in diseased human muscle cells obtained from biopsies of DMD patients. A Phase I/II clinical trial is scheduled to start by the end of 2012.
AMT has received an Innovation Credit of up to € 4 million from the Dutch government to support the development of AMT’s gene therapy treatment for Duchenne Muscular Dystrophy (DMD). The credit is granted by SenterNovem, an agency of the Dutch Ministry of Economic affairs.
“It is exceptional that we have been able to reveal the promise of this therapy to the FDA in this early stage of the development. We believe our proven adenoassociated viral vector technology used in all our gene therapy products provides a distinct advantage. AMT has successfully conducted three clinical trials with its lead product Glybera that employs this technology, confirming that AAV-based delivery technology is safe and efficacious,” noted Jörn Aldag, CEO of Amsterdam Molecular Therapeutics.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.
Last viewed contents
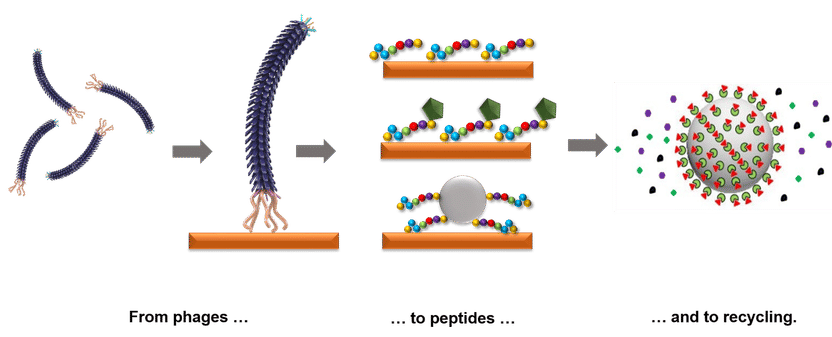
‘Bio-fishing’ for rare earths - How protein fragments can be used for the recycling of electronics waste
Oncgnostics starts international sales partnership with EUROIMMUN
Replacing damaged ‘hair’ cells may help treat hearing loss
Catabiosis
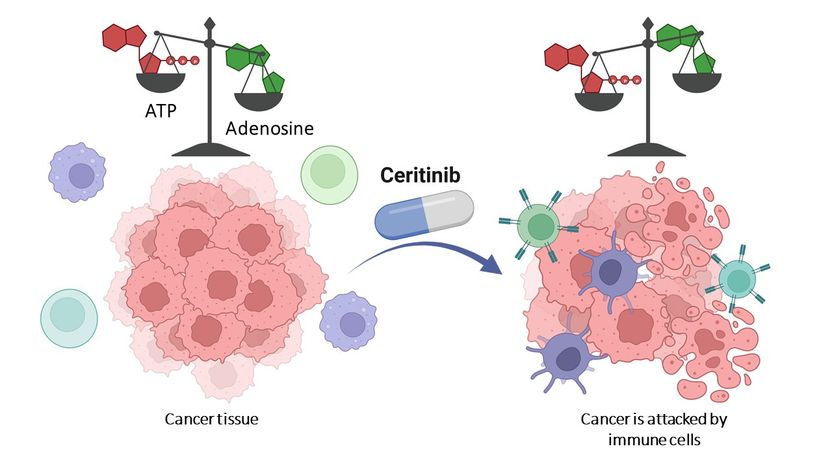
Therapeutic drug renders cancer cell weapon harmless - Already approved drug could pave way to new pharmaceuticals