Positive Study Findings from Lixisenatide in First Phase III GetGoal Trial for Type 2 Diabetes
Zealand Pharma A/S announced that the positive study findings from the first Phase III GetGoal clinical trial program with lixisenatide will be presented by Zealand Pharma's partner, sanofi-aventis, at the European Association for the Study of Diabetes (EASD) 46th Annual Meeting in Stockholm, Sweden.
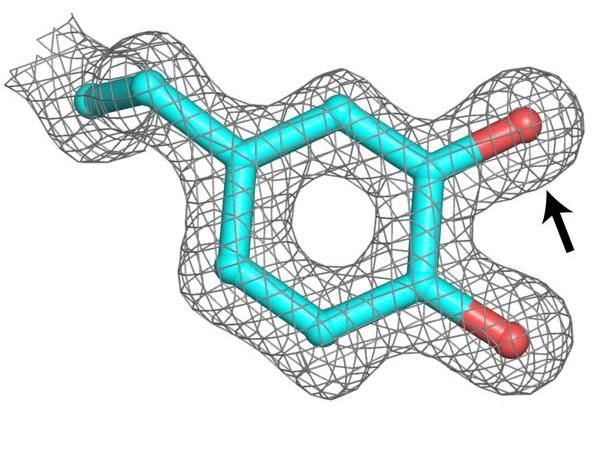
The study has assessed the efficacy and safety of lixisenatide, a once-daily GLP-1 receptor agonist invented by Zealand Pharma, as a monotherapy in patients with type 2 diabetes. The trial was designed as a 12-week, randomized, double-blind, multicentre, phase 3 study with 361 patients participating in total. The full Phase III GetGoal clinical program includes 10 trials and more than 4,000 patients.
Highlights of the Lixisenatide announcement issued by sanofi-aventis are:
- Significant improvement in glycemic control versus placebo with a pronounced postprandial effect
- Significant reduction in A1C levels versus placebo (p<0.0001)
- Mean decreases in body weight observed in all groups
- Well tolerated and acceptable safety profile (only one serious treatment-emergent adverse event (TEAE) occurred in the lixisenatide group versus five in the placebo group. Nausea was the most frequent TEAE with lixisenatide
- Study investigator said results demonstrate lixisenatide as a once daily GLP-1 agent and that the data provided a rationale for investigating the combined effect of lixisenatide and long acting insulins in patients with type 2 diabetes
Commenting on the announcement David Solomon, Chief Executive Officer and President of Zealand Pharma, said: "We are delighted that lixisenatide has shown significant efficacy as well as safety data in this GetGoal Phase III clinical trial. These study findings demonstrate the potential of lixisenatide for the treatment of type-2 diabetes. Zealand Pharma's scientists are pioneers in peptide drug innovation and development and we look forward to further clinical trial read-outs from the GetGoal study."
Most read news
Organizations
Other news from the department price development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

FSU research links common sweetener with anxiety

University of Ottawa's Faculty of Medicine team reveals underpinnings of how motor memory forms - A research team led by Dr. Simon Chen offers new and valuable insights into an enduring mystery of neuroscience
Takeda Announces Top Line Results from Phase 2 Study of Investigational Compound, TAK-442 for the patients with ACS
List_of_subjects_in_Gray's_Anatomy:_V._Angiology