Antisoma's phase III trial of AS1413 completes patient enrolment
Antisoma plc announces that the ACCEDE phase III trial of AS1413 (amonafide L-malate) in secondary acute myeloid leukaemia (secondary AML) is now fully enrolled. Data from the trial are expected in the first half of 2011, with filings for marketing authorisations to follow if these are positive.
ACCEDE is a single pivotal, randomised, controlled trial in which a regimen of AS1413 and cytarabine is compared with standard AML remission-induction therapy of daunorubicin and cytarabine ('7+3'). The primary endpoint of the study is the rate of complete remission with or without recovery of normal blood counts.
Over 420 patients from 22 countries have been included, making it the largest prospective trial ever conducted in patients with secondary AML.
Secondary AML is a significant subgroup of AML that develops from prior myelodysplastic syndrome (MDS) or follows treatment of another cancer with chemotherapy or radiotherapy. The disease is often multi-drug resistant and responds poorly to currently available therapies. A key feature of AS1413, and a potential advantage over many current AML treatments, is the drug's ability to evade multi-drug resistance mechanisms.
Glyn Edwards, CEO of Antisoma, said: "Completion of enrolment in the phase III trial is a critical milestone in the development of AS1413, and puts us on track to see the outcome in the near future. I would like to thank all the patients and physicians who have joined with us in seeking to improve the treatment of secondary AML."
AS1413 has orphan drug status in both the U.S. and the E.U. for the treatment of AML and recently received Fast Track status from the U.S. FDA for the treatment of secondary AML.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
List_of_subjects_in_Gray's_Anatomy:_VI._The_Arteries
Medicines Agency gives go-ahead for new Pfizer logistic concept in Denmark - Customised solution: the trans-o-flex Logistics Group supplies all Danish hospitals and wholesalers within 24 hours after collecting the goods in Germany
Valneva Announces Start of Phase II Clinical Trial of its Clostridium difficile vaccine candidate
Pantothenate_kinase-associated_neurodegeneration
Affimed enrolls first patients in Phase I Hodgkin's Lymphoma Study
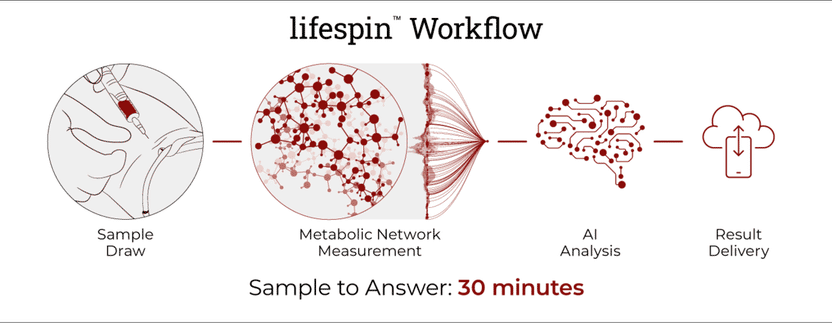
Lifespin secures bridge financing - Artificial intelligence to determine health status and diagnose diseases
Price competition for generic drugs linked to increase in manufacturing-related recalls
TranScrip Partners expands further with two new senior appointments - Julia Lloyd-Parks and Mark Watling join the organisation as Senior Partners
Norman-Roberts_syndrome
List_of_subjects_in_Gray's_Anatomy:_III._Syndesmology
What is a laboratory mouse? Jackson, UNC researchers reveal the details