Antisoma's phase III trial of AS1413 completes patient enrolment
Antisoma plc announces that the ACCEDE phase III trial of AS1413 (amonafide L-malate) in secondary acute myeloid leukaemia (secondary AML) is now fully enrolled. Data from the trial are expected in the first half of 2011, with filings for marketing authorisations to follow if these are positive.
ACCEDE is a single pivotal, randomised, controlled trial in which a regimen of AS1413 and cytarabine is compared with standard AML remission-induction therapy of daunorubicin and cytarabine ('7+3'). The primary endpoint of the study is the rate of complete remission with or without recovery of normal blood counts.
Over 420 patients from 22 countries have been included, making it the largest prospective trial ever conducted in patients with secondary AML.
Secondary AML is a significant subgroup of AML that develops from prior myelodysplastic syndrome (MDS) or follows treatment of another cancer with chemotherapy or radiotherapy. The disease is often multi-drug resistant and responds poorly to currently available therapies. A key feature of AS1413, and a potential advantage over many current AML treatments, is the drug's ability to evade multi-drug resistance mechanisms.
Glyn Edwards, CEO of Antisoma, said: "Completion of enrolment in the phase III trial is a critical milestone in the development of AS1413, and puts us on track to see the outcome in the near future. I would like to thank all the patients and physicians who have joined with us in seeking to improve the treatment of secondary AML."
AS1413 has orphan drug status in both the U.S. and the E.U. for the treatment of AML and recently received Fast Track status from the U.S. FDA for the treatment of secondary AML.
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
Last viewed contents
EMBO recognizes 63 researchers for advances in life sciences
Sensitizing tumor response to cancer therapy
PANATecs GmbH Appoints Rainer Baule, Chairman of the Board of Fresenius Kabi AG, to the Advisory Board

Siegfried begins construction of new large-scale production plant in Minden (DE) - Total investment of up to CHF 100 million

Wyss Institute at Harvard University launches SPEAR Bio - Spear Bio is working towards commercializing its first assay, which accurately assesses levels of SARS-CoV-2-neutralizing antibodies in dried-blood spot samples
HIV infection: the brevity of the right moment
Study sheds light on treating cancer - Using light to target and kill cancer cells alternatively without surgery
First structural map of cystic fibrosis protein sheds light on how mutations cause disease
Scientists identify gene that predicts post-surgical survival from brain metastasis of breast cancer patients
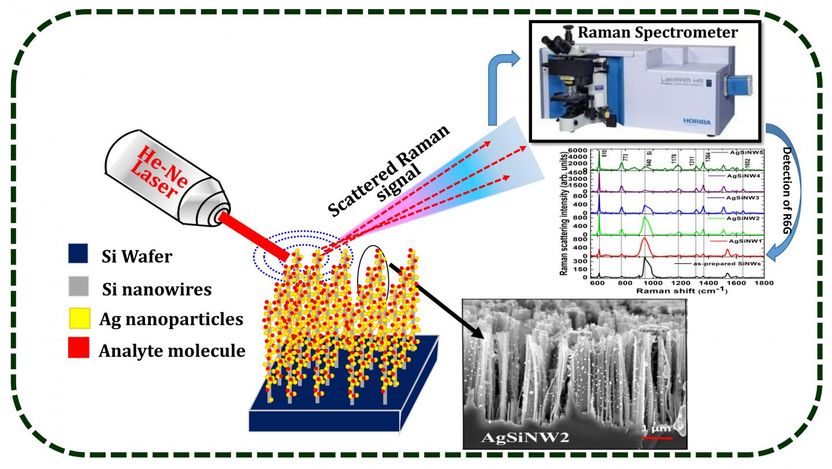
Silver nanoparticles take spectroscopy to new dimension - A new way of organizing nanostructures has boosted Raman signals by a hundred thousand times to better identify and characterize different molecules

Software revolution for clinical trials - Health Tech Start-up Carelane Raises €800,000 in Pre-Seed Funding