ThromboGenics Presents Positive Pooled Results from the MIVI-TRUST Phase III Program
ThromboGenics NV announced that the pooled results from the successful microplasmin MIVI-TRUST Phase III program were presented at the EURETINA (European Society of Retina Specialists) Congress in Paris, France. The MIVI-TRUST program is the largest interventional clinical program ever performed to specifically evaluate the vitreoretinal interface in patients with retinal disorders, recruiting a total of 652 patients at 90 centers across the U.S. and Europe.
The pooled results of the TG-MV-006 and TG-MV-007 Phase III trials were presented by Prof. Peter Stalmans (University Hospitals Leuven, Belgium). These results demonstrate the potential of microplasmin to transform the treatment of a range of retinal disorders.
The Phase III program showed that microplasmin:
- Was successful in resolving vitreomacular adhesion (VMA)
- Was able to cure full thickness macular hole (FTMH) without the need for surgery
- Delivered an improvement in the vision of patients without the need for surgery
- Was safe and well tolerated
Both the TG-MV-006 and TG-MV-007 trials met the primary endpoint, achieving a statistically significant improvement in the resolution of VMA. The pooled results from the MIVI-TRUST program showed that 26.4% of the 465 microplasmin treated patients achieved resolution of their VMA at 28 days, compared to 10.2% of the 182 patients who received a placebo injection, a highly statistically significant result (p=0.000002).
In patients without epiretinal membrane, microplasmin was shown to be even more effective, with 37.4% of 270 patients achieving nonsurgical resolution of their VMA at 28 days, compared to 14.3% of 119 placebo treated patients (p=0.000003). Epiretinal membrane is a layer of scar tissue which builds up on the macula, making it more difficult to achieve resolution of VMA without surgical intervention. Epiretinal membrane can be easily identified using Optical Coherence Tomography (OCT).
The MIVI-TRUST program’s pooled results also highlighted microplasmin’s impressive effect in patients diagnosed with FTMH. In this group, 40.6% of the 106 patients saw closure of their FTMH at 28 days following a single 125μg injection of microplasmin without the need for a vitrectomy. This compares with 10.6% of the 47 patients in the placebo group (p= 0.00015). The closure of FTMH also led to microplasmin treated patients experiencing a significant improvement in their visual acuity (VA) compared to baseline.
Prof. Stalmans also presented an analysis of the pooled visual acuity data from the Phase III program. This showed that at the end of the six month study period, 23.7% of the microplasmin treated patients had achieved at least a 10 letter (2 lines) improvement in VA without the need for vitrectomy. This compares to only 11.2% of the patients who received a placebo injection (p=0.0002). Within the microplasmin treated population, 9.7% of patients achieved a 15 letter (3 lines) improvement in their visual acuity without the need for vitrectomy, compared to just 3.7% of the placebo patients (p=0.01). In addition, microplasmin treated patients showed an improved Quality of Life when compared to placebo, based on the VFQ-25 (the National Eye Institute Visual Functioning Questionnaire) results.
The pooled results also confirmed that microplasmin was generally safe and well tolerated. There was no evidence of an increased risk of retinal tear or detachment.
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Chloralose
Impression_(dental)
Medivir announces new studies in phase III program for TMC435 - Study in previous non-responder Hepatitis C genotype-1 infected patients
Amyotrophic_lateral_sclerosis
Ferriman-Gallwey_score
GE Healthcare completes acquisition of Whatman plc
Osmotic_demyelination_syndrome
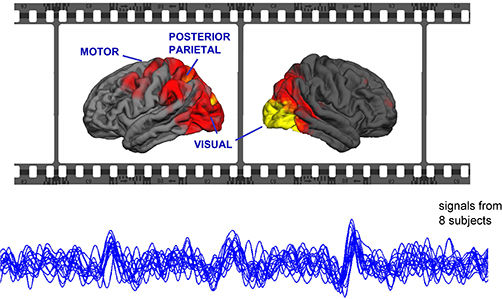
Movies synchronize brains - When we watch a movie, our brains react to it immediately in a way similar to other people's brains.
Innovative genetic and cellular techniques help identify multiple disease targets
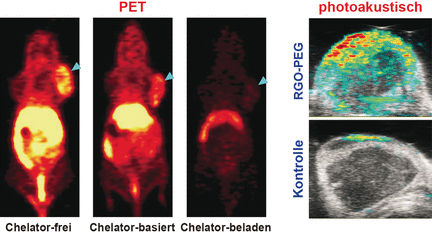
Direct radiolabeling of nanomaterials
Research will speed tracing bacteria behind salmonella outbreaks