Addex Partner Completes ADX71149 Phase I Program
Addex Pharmaceuticals Ltd announced that the phase I studies required to commence Phase II testing of ADX71149 have been satisfactorily completed. Subject to regulatory authorizations, phase IIa studies in schizophrenia and anxiety, are scheduled to start enrolling patients in the first quarter of 2011.
The Phase I program was conducted by Johnson & Johnson Pharmaceutical Research and Development (J&JPRD) on behalf of Ortho-McNeil-Janssen Pharmaceuticals, Inc. (OMJPI) and included more than five studies in normal human volunteers, including: single ascending dose; multiple ascending dose; food & gender; ketamine challenge (a model of psychosis); and anxiety challenge.
In 2005, Addex Pharmaceuticals announced it had entered an exclusive worldwide research collaboration and license agreement with OMJPI to discover, develop and commercialize novel allosteric compounds for the treatment of anxiety, depression, schizophrenia and Alzheimer's disease. The initial drug discovery research was conducted both at Addex and at J&JPRD. Under the terms of the agreement, OMJPI paid Addex a €3 million upfront fee and research funding for 2 years. In addition, Addex is eligible for up to a total of €112 million subject to successful completion of development and regulatory milestones. In addition, Addex is eligible for low double-digit royalties on sales of ADX71149, subject to regulatory approval and successful commercial launch.
“ADX71149 is a truly innovative, potentially life changing drug candidate for patients suffering from schizophrenia, anxiety and possibly other disorders,” CEO Vincent Mutel said. “The Phase I program demonstrates that ADX71149 tolerability and safety monitoring parameters were within acceptable limits and support continued study in patients.”
ADX71149 is an mGluR2 positive allosteric modulator (PAM) being developed by OMJPI. Activation of mGluR2 is a clinically validated mechanism in both schizophrenia and anxiety.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents

Changes in Merck Executive Board - Belén Garijo appointed Vice Chairman of the Executive Board and Deputy CEO
Somerled
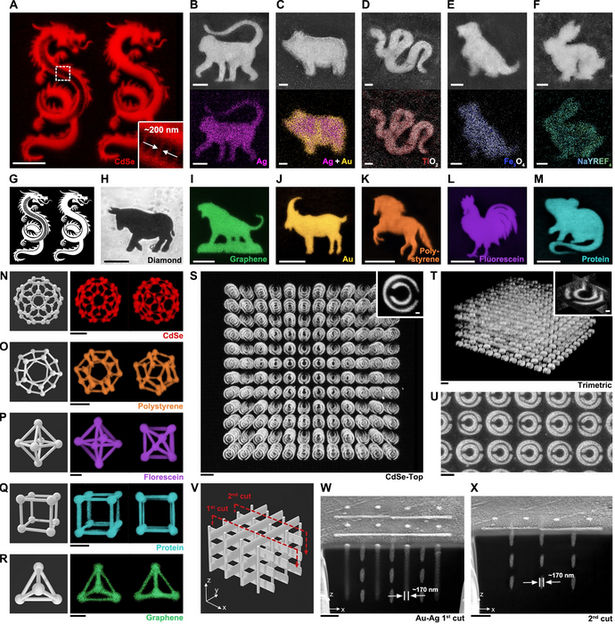
Shrinking hydrogels enlarge nanofabrication options - Researchers print intricate, 2D and 3D patterns for use in in biotechnology, photonics or nanodevices
Metabolomics
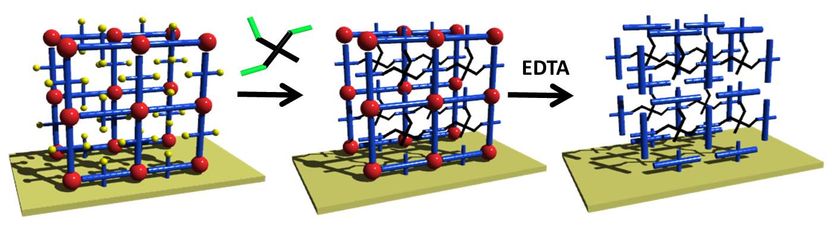
Polymer Coatings Based on Molecular Structures - Researchers Developing a Novel Gel for Biological and Medical Applications