Sandoz leads the way with first generic version of 'gold standard' anti-thrombotic Lovenox
Sandoz announced the introduction of enoxaparin sodium injection, the first generic version of Lovenox®, in the US. Product shipment began immediately following approval by the US food and Drug Administration (FDA).
According to IMS data enoxaparin sodium injection is the best-selling hospital medicine in the US, and has been described as the 'gold standard' for anti-thrombotic treatments. Lovenox®, the reference product, recorded US sales of USD 2.7 billion in 2009 and has been used to treat an estimated 200 million patients worldwide since it was launched.
"Sandoz is the first company to launch generic enoxaparin sodium in the US, delivering on our strategy of being first-to-market with key products, and underscoring our leadership in differentiated products," said Jeff George, global head of Sandoz. "We welcome the FDA decision to approve our enoxaparin application, and are now looking forward to significantly increasing patient and payor access to this vital medicine, by providing a high-quality, more affordable version."
The Sandoz product, developed in collaboration with Momenta Pharmaceuticals, Inc., is indicated for use in prophylaxis and for treatment of deep vein thrombosis, prophylaxis of ischemic complications of unstable angina and non-Q-wave myocardial infarction, and the treatment of acute ST-segment elevation myocardial infarction [STEMI].
Most read news
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Isothiocyanate
Blood
Category:Organ_systems
Centers_of_Biomedical_Research_Excellence
SYGNIS is granted the European and US patent for a very versatile and efficient drug screening platform
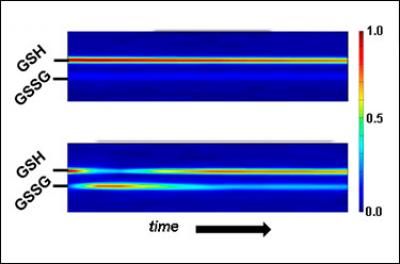
Response to oxidation in live cells
Anterior_Spinal_Syndrome
5-MeO-DMT
Peter_McWilliams
YM Biosciences Reports Additional Results From Nimotuzumab Phase III Study in Children With Glioma
Dementia