Cardio3 BioSciences Reports Positive Three-Month Data from its Clinical Trial of C-Cure in Heart Failure
Cardio3 BioSciences announced positive safety data and preliminary efficacy results from its clinical trial of C-Cure®, a stem cell therapy for heart failure. Results showed C-Cure to have a very good safety profile with no adverse events related to C-Cure, as assessed by an independent board. The study is also examining a number of measures of efficacy. With three month follow up data in-hand, Cardio3 BioSciences has observed positive and encouraging trends in a number of physiological and clinical parameters. Meaningful differences were seen in ventricular size, ejection fraction and other measures of heart muscle activity in C-Cure treated patients when compared to control and to baseline. Partial data from a paired analysis of patients at six-months follow-up is suggestive of these beneficial trends being reinforced over time. Cardio3 Biosciences intends to publish the study results once the full six month dataset is available and has been analysed.
Dr Christian Homsy, CEO of Cardio3 BioSciences said: “The data so far from our trial is very encouraging. We showed that C-Cure is safe at three months. We also saw positive trends in other measures that suggest that C-Cure, as anticipated from animal model data, is acting on heart muscle in a way that could yield important clinical benefits. We now look forward to seeing the full six-month follow-up data and completing the analysis of the trial.”
The current C-Cure study is a randomised, prospective, multi-center trial to evaluate the safety and efficacy of C-Cure beyond optimal clinical care in patients with heart failure. It recruited 45 patients in Belgium and Serbia. The primary end point of the trial is change in left ventricular ejection fraction (a measure of how well the heart is functioning) at six months post treatment.
Using the insights from the trial and input from regulators in Europe and the US, Cardio3 BioSciences is now designing a pivotal clinical trial program for C-Cure expected to start in 2011. With the Phase II stage completed and to allow for potential modifications to the trial protocol, Cardio3 BioSciences will not continue recruitment into the existing trial but will continue to gather all data for the six month analysis.
Most read news
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
UCLA researchers discover new molecular pathway for targeting cancer, disease
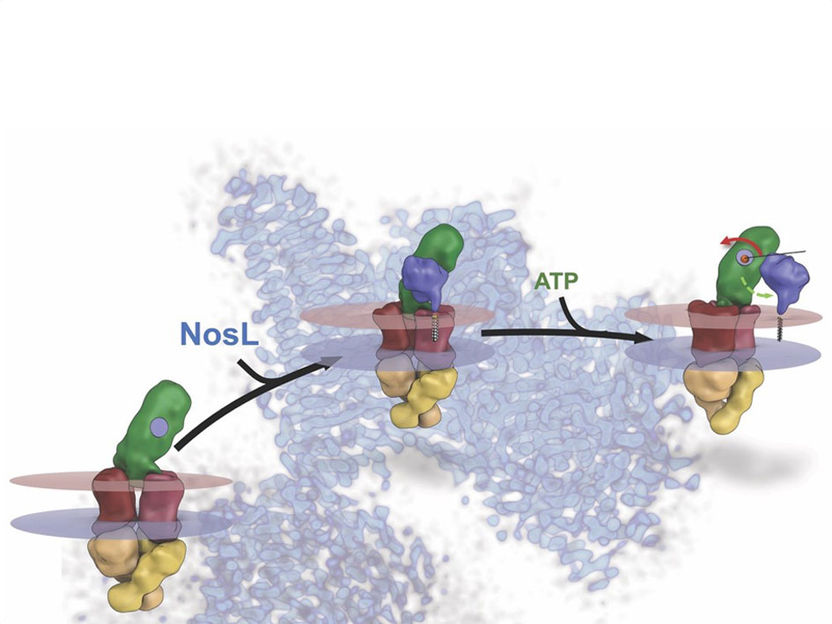
A molecular machine at work - Researchers unravel the assembly of an enzyme that detoxifies the greenhouse gas N₂O
How one strain of MRSA becomes resistant to last-line antibiotic
Ablynx Successfully Generates Nanobodies Against an Ion Channel and Announces New GPCR Programme
Emotional_Freedom_Techniques
Santhera Announces Intent To File Marketing Authorization Application for Leber's Hereditary Optic Neuropathy
Janssen-Cilag International NV withdraws its application for an extension of the indication for Velcade (bortezomib)
Hip_fracture_treatment