Basilea provides update on phase III program for the antifungal isavuconazole
Basilea Pharmaceutica Ltd. announced that its partner Astellas Pharma Inc.has initiated the formal process to allow continuation of patient recruitment into the phase III clinical studies of isavuconazole for the treatment of life-threatening invasive fungal infections. Certain study protocol amendments have been implemented and the phase III program will be expanded to include more patients.
Following transfer completion of the Investigational New Drug (IND) application and clinical trial sponsorship internationally to Astellas, the process to re-open phase III clinical sites for the isavuconazole trials has commenced. The safety, tolerability and efficacy of isavuconazole is being investigated in three phase III studies, one targeting yeast infections (candidemia and other invasive Candida infections), one mold infections (invasive aspergillosis) and a third trial including rare molds infections and renally impaired patients with aspergillosis.
Following the review of the phase III clinical program, Astellas and Basilea have implemented certain study protocol amendments to the invasive aspergillosis study in order to account for changes in clinical practice, the evolving regulatory environment and ongoing advancements in clinical diagnostics. The joint development program envisages increasing the proportion of patients to be recruited with probable and proven Aspergillus infections as well as more extensive use of the galactomannan diagnostic test.
Subject to local Ethic Committees' approvals, treatment of first new patients in the phase III program is anticipated in Q3 2010. Given the anticipated timing for initiation of all clinical sites and estimated recruitment rates, patient recruitment is anticipated to be completed in 2012 and first trial results from the phase III program are expected in 2013.
"The implementation of these protocol amendments that allow for a swift continuation of patient recruitment and the anticipated expansion of patient numbers underscore Astellas' and our strong commitment to pursue the best development path for this innovative treatment," said Professor Achim Kaufhold, M.D., Chief Medical Officer, Basilea Pharmaceutica International Ltd. "Despite the change to the initially anticipated timelines, we believe that the envisaged changes will further strengthen the competitive positioning of isavuconazole in an evolving medical field."
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
More news from our other portals
See the theme worlds for related content
Last viewed contents
Dental_restoration
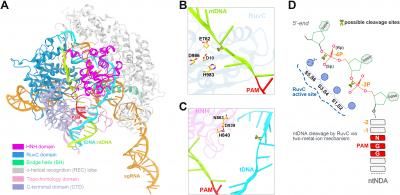
Catching CRISPR in action
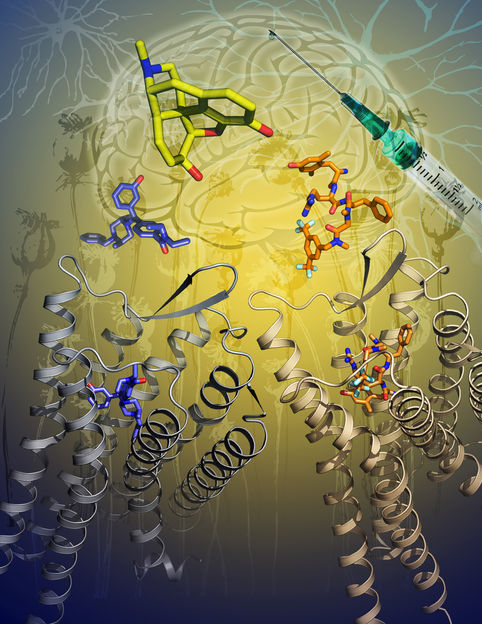
Activation of opioid receptor uncovered

miDiagnostics - Heverlee, Belgium
Large bacterial populations develop stronger resistance to antibiotics - In large populations, mutants that evolve relatively late resist antibiotic treatment more effectively, while smaller populations rely on less effective mutations that appear at an earlier point in time
Abdominal_pain
Cholesterol an Important Piece of the Puzzle for Fat-Burning
Category:Vascular_disorders