Visualising the Invisible: Active Sites in Antibacterial Nanomaterials
Fluorescence lifetime microscopy has great potential to systematically enhance the effectivenes of antibacterial nanomaterials
Interest in antibacterial nanomaterials has surged due to the growing threat of antibiotic-resistant microbes. A particularly promising strategy involves the use of nanomaterials that generate reactive oxygen species upon exposure to light, effectively combating microorganisms. However, a key challenge lies in translating the properties observed at the molecular level to the material as a whole. Researchers at the University of Duisburg-Essen, led by Junior Professor Dr. Anzhela Galstyan, have made significant progress in addressing this challenge. Using fluorescence lifetime microscopy, they visualised the active sites of these materials for the first time, enabling them to establish correlations between activity and material properties. Their findings were recently published in Angewandte Chemie.
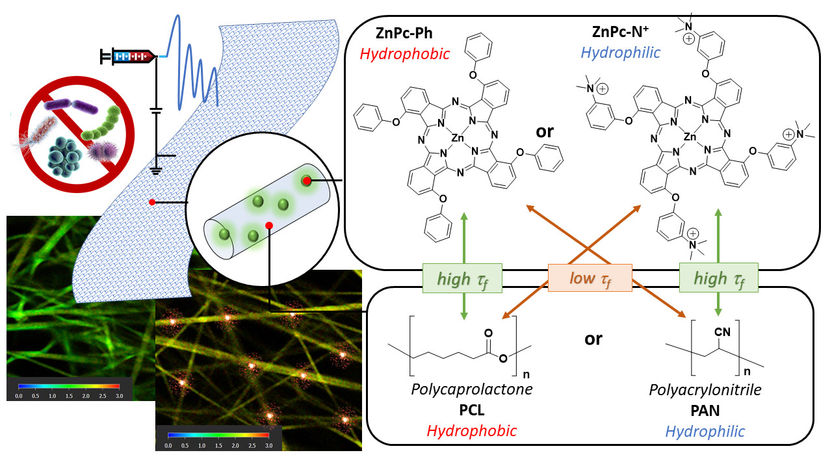
left side: FLIM images show the difference between an active and an inactive membrane. In the active membrane, red areas highlight active sites where the photosensitizer (PS) remains in an excited state, signifying high activity. In the picture on the right: Schematic representation of the antibacterial membrane with the chemical structures of the (PS) and polymers used.
Universität Duisburg-Essen
Bacteria can also be fought without conventional antibiotics. One option is to use photodynamic inactivation. In this process, special light-sensitive molecules, known as photosensitisers, are activated by irradiation with light. These generate reactive oxygen species that inactivate bacteria by attacking their proteins, DNA and parts of the cell walls.
‘We utilise this principle and integrate photoactive molecules into nanofibre membranes in order to use them for water treatment’, explains Prof. Dr Galstyan. However, highly active photosensitisers on a molecular level do not automatically result in equally high-performing membrane materials. Galstyan's team therefore applied fluorescence lifetime microscopy (FLIM) to visualise the distribution of photosensitisers within antibacterial nanomembranes. The fluorescence lifetime refers to the period during which light-sensitive molecules remain excited after activation—an indicator that allows conclusions to be drawn about the amount of reactive oxygen species generated.
When analysing the FLIM images, the researchers observed that the distribution of sensitisers in the nanofibres studied varied significantly. For the study, polymer-based membranes were fabricated using electrospinning techniques, combining different surface properties (hydrophobic and hydrophilic). ‘We observed the highest antibacterial activity in a nanofibre that incorporated the hydrophilic, water-attracting ZnPc−N+ as a photosensitiser,’ reports Galstyan.
The FLIM images explain this observation: ‘At the interface between water and the membrane, there is a high density of active sites with extended lifetimes. The hydrophilic photosensitisers tend to orient themselves towards the water-attracting phase and preferentially accumulate at the material-water interface,’ explains the junior professor for nanomaterials in aquatic systems.
‘Utilising FLIM imaging, we can not only identify the position of active sites within the nanofibres but also establish a direct link between material structure and antibacterial activity,’ Galstyan summarises. For the future development of antibacterial nanomaterials, she sees great potential in fluorescence lifetime microscopy to systematically enhance the effectiveness of such materials.
Original publication
Other news from the department science
Most read news
More news from our other portals
See the theme worlds for related content
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.
Topic world Fluorescence microscopy
Fluorescence microscopy has revolutionized life sciences, biotechnology and pharmaceuticals. With its ability to visualize specific molecules and structures in cells and tissues through fluorescent markers, it offers unique insights at the molecular and cellular level. With its high sensitivity and resolution, fluorescence microscopy facilitates the understanding of complex biological processes and drives innovation in therapy and diagnostics.