Markers in iPSC quality control – a new approach to enhance standardization
“Machine learning is an attractive tool to streamline processes”
Human induced pluripotent stem cells (iPSCs) have a great potential for scientific and medical applications. They are used in research laboratories to model human diseases, helping to uncover underlying mechanisms and developing new therapies. Additionally, they are being tested in clinical trials as a solution for regenerative therapies targeting diseases like Parkinson’s, with the goal of restoring function and improving patient outcomes. iPSCs can be obtained from somatic cells, such as those from skin or blood of adult donors, by reprogramming these cells with the help of pluripotency factors, transforming them into cells with embryonic stem cell-like properties. These iPSCs can replicate indefinitely and develop into different tissues making them valuable for research and potentially for personalized regenerative medicine. At the same time, they help to reduce the number of laboratory animals in research.

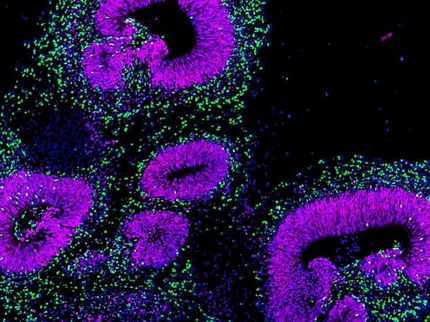
A fluorescent microscopy image of a branching lung organoid which was generated using human induced pluripotent stem cells. The lung epithelial cell boundaries are stained in green. Nuclei are stained in blue. Scale bar: 75 µm.
IUF / Jochen Dobner
While quality controls for iPSCs are essential, they are not yet fully standardized. These quality controls include the assessment of morphology, nuclear and mitochondrial genome integrity, and pluripotency, i.e., their capability to differentiate into the three primary germ layers endoderm, ectoderm, and mesoderm. Thus, there is a pressing need for testing methods that are easy, fast, and cost-effective. In former studies the lab of Dr. Andrea Rossi at the IUF – Leibniz Research Institute for Environmental Medicine already looked into mitochondrial genome integrity. A study recently published in Nature Communications by Dobner et al. focuses on pluripotency. The authors used long-read nanopore transcriptome sequencing to discover 172 genes linked to cell states not covered by current guidelines. They validated 12 genes by qPCR (quantitative polymerase chain reaction) as unique markers for specific cell fates: undifferentiated pluripotent iPSCs (CNMD, NANOG, SPP1), endoderm (CER1, EOMES, GATA6), mesoderm (APLNR, HAND1, HOXB7), and ectoderm (HES5, PAMR1, PAX6). Based on these selected genes, they developed a machine learning-based scoring system, “hiPSCore”, which was trained on 15 iPSC lines and validated on additional 10 iPSC lines. The hiPSCore accurately classifies pluripotent and differentiated cells and predicts their potential to become specialized 2D cells and 3D organoids.
“We always strive to improve the methods which we are using”, explains Dr. Andrea Rossi, group leader at the IUF, the approach of the study. “We plan to further develop quality controls for standardized use of iPSCs within the next years.” Dr. Jochen Dobner, first author of the study adds: “Machine learning is an attractive tool to streamline processes. Our developed hiPSCore improves iPSC testing by reducing time, subjectivity, and resource use, thus enhancing iPSC quality for scientific and medical applications.”