Powering enzymes with light to make ammonia
Scientists examine how molecular systems made of nanocrystals and proteins support the production of ammonia using light.
Advertisement
The Earth’s atmosphere contains large amounts of nitrogen in the form of dinitrogen gas (N2). Converting N2 to ammonia (NH3) is critical for making the fertilizer needed for agriculture. Currently, ammonia production requires 2% of the global energy and generates significant Greenhouse Gases. In nature, the nitrogenase enzyme can catalyze ammonia production by using the energy stored in adenosine triphosphate (ATP) to drive the reaction. ATP is a natural molecule found in all forms of life. It is possible to replace ATP with sunlight energy for a low energy process that does not produce greenhouse gases. However, researchers are still developing these sunlight-based processes. In this research, scientists created a unique biohybrid that couples nanocrystals to nitrogenase. The nanocrystals use sunlight to transfer charge to the enzymes and complete the reaction. The research identified the properties of nanocrystals for binding to nitrogenase, helping the scientists gain new insights into this complex NH3 production reaction.
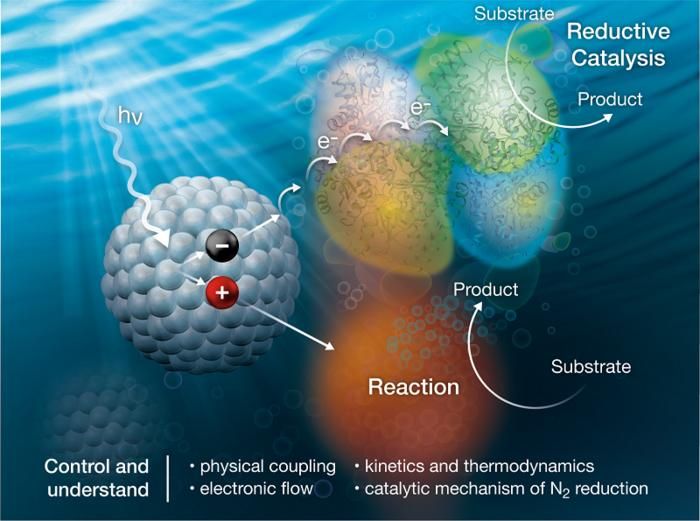
Nanocrystals (left) capture light (hv) and then transfer electrons (e-) to nitrogenase enzymes (upper right) to convert dinitrogen (N2) to ammonia (NH3). A sacrificial reaction (bottom right) completes the process.
Image courtesy of Alfred Hicks, National Renewable Energy Laboratory.
This biohybrid approach uses sunlight to drive the energy-demanding conversion reactions that can mitigate the co-production of greenhouse gases. The standard approach to making ammonia is the Haber-Bosch process. This process produces about 150 million metric tons (MmT) of ammonia per year but requires large amounts of energy and also produces about 280 MmT of carbon dioxide (CO2). The new process uses sunlight to catalyze NH3 production without generating CO2. It is also an attractive way to produce NH3 fertilizers close to where they will be used, minimizing CO2 emissions from shipping to farms. Making this process a reality requires understanding how to couple sunlight to drive the reaction.
To produce ammonia using sunlight, research scientists developed a biohybrid system composed of nanocrystals and the enzyme Mo-nitrogenase. This enzyme has a unique metal cluster, termed the FeMo-cofactor, that requires eight electrons and eight protons to reduce N2 to ammonia. The researchers used this nanocrystal/enzyme system to determine how to direct photogenerated electrons to the FeMo-cofactor and to study the related mechanism. For the system to rely on light, the nanoparticle and enzyme must be chemically compatible and form a stable reaction complex. This research explored how to make nanoparticles that bind to the enzyme.
This approach provides insight into how to synthetically tune nanocrystals to bind enzymes and to transfer charge selectively. Taking advantage of this progress, researchers can study the process in detail. In the frozen state, the FeMo-cofactor reaction intermediates can be trapped and analyzed in detail by electron paramagnetic resonance spectroscopy techniques. This technical foundation enables researchers to identify reaction intermediates, the activation energies of the reaction steps, and evolution of a kinetic model of the N2 reduction reaction.























































