Revolution in blood donation? Enzymes open new path to universal donor blood
Researchers have discovered enzymes that remove hindrances that stood in the way of developing universal donor blood
The quest to develop universal donor blood has taken a decisive step forward. Researchers at Technical University of Denmark (DTU) and Lund University have discovered enzymes that, when mixed with red blood cells, are able to remove specific sugars that make up the A and B antigens in the human ABO blood groups. The results have been published in the scientific journal Nature Microbiology.
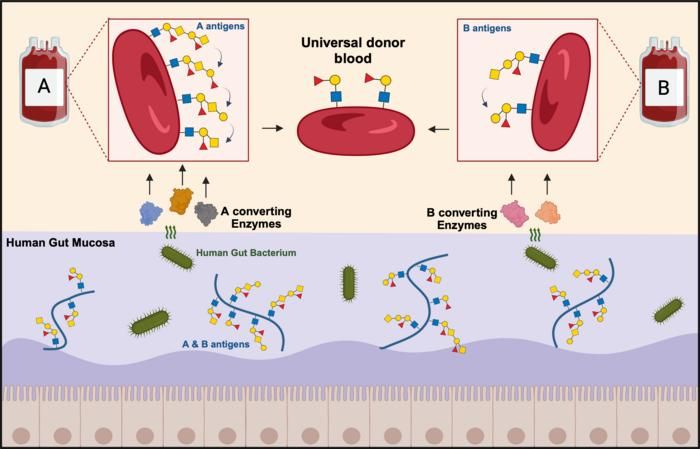
The ABO blood group antigens found on the surface of red blood cells are also found on the mucosal layer that lines the surface of the gut. Researchers have harnessed a specialized human gut bacterium and its ability to use these antigens as nutrients to discover and develop two enzyme mixtures that convert group A and B red blood cells into universal donor blood.
Graphic: Mathias Jensen, postdoc at DTU.
"For the first time, the new enzyme cocktails not only remove the well-described A and B antigens, but also extended variants previously not recognized as problematic for transfusion safety. We are close to being able to produce universal blood from group B donors, while there is still work to be done to convert the more complex group A blood. Our focus is now to investigate in detail if there are additional obstacles and how we can improve our enzymes to reach the ultimate goal of universal blood production," says Professor Maher Abou Hachem, who is the study leader at DTU and one of the senior scientists behind the discovery.
He states that the discovery is the result of combining the expertise of DTU researchers in enzymes from the human gut microbiota and Lund University researchers in carbohydrate-based blood groups and transfusion medicine.
High demand for donor blood
Human red blood cells carry specific complex sugars structures (antigens) that define the four ABO blood groups A, B, AB and O. These antigens control compatibility between donors and recipients for safe blood transfusion and organ transplantation. Donor blood is screened for disease markers and the main blood groups. It can then be stored refrigerated for up to 42 days.
The need for donor blood is high due to the elderly making up a larger proportion of the population and more patients undergoing blood-intensive medical procedures. Successfully converting A or B blood types into ABO universal donor blood can markedly reduce the logistics and costs currently associated with storing four different blood types. In addition, the development of universal donor blood will lead to an increased supply of donor blood by reducing the waste of blood approaching its expiry date.
The reason why it is necessary to remove the A and B antigens to create universal donor blood is because they can trigger life-threatening immune reactions when transfused into non-matched recipients.
The concept of using enzymes to generate universal donor blood was introduced more than 40 years ago. Since then, higher efficiency enzymes to remove the A and B antigens were discovered, but researchers are still not able to explain or abolish all immune reactions related to the blood, and therefore these enzymes are still not used in clinical practice.
Enzymes from the gut
The research groups from DTU and Lund University have gone new ways to find enzymes that can remove both the A and B blood antigens and the sugars that block them. The research teams discovered new mixtures of enzymes from the human gut bacterium Akkermansia muciniphila that feeds by breaking down the mucus, which covers the surface of the gut. It turns out that these enzymes are exceptionally efficient, as the complex sugars at the surface of the intestinal mucosa share chemical resemblance with those found at the surface of blood cells.
"What is special about the mucosa is that bacteria, which are able to live on this material, often have tailor-made enzymes to break down mucosal sugar structures, which include blood group ABO antigens. This hypothesis turned out to be correct," says Maher Abou Hachem.
The researchers in this study tested 24 enzymes, which they used to process hundreds of blood samples.
"Universal blood will create a more efficient utilization of donor blood, and also avoid giving ABO-mismatched transfusions by mistake, which can otherwise lead to potentially fatal consequences in the recipient. When we can create ABO-universal donor blood, we will simplify the logistics of transporting and administering safe blood products, while at the same time minimizing blood waste" says Professor Martin L. Olsson, the leader of the study at Lund University.
The researchers from DTU and Lund University have applied for a patent on the new enzymes and the method for enzyme treatment and expect to make further progress on this in their new joint project over the next three and a half years. If successful, the concept needs to be tested in controlled patient trials before this can be considered for commercial production and clinical use.
The initial research project is funded by the Independent Research Fund Denmark (Technology and Production Sciences, FTP), the Swedish Research Council, ALF grants from the Swedish government and county councils as well as the Knut and Alice Wallenberg Foundation and Research Fund Denmark, Natural Sciences, FNU), while the new continued project is funded by the Novo Nordisk Foundation, Interdisciplinary Synergy Programme.
Original publication
Mathias Jensen, Linn Stenfelt, Jennifer Ricci Hagman, Michael Jakob Pichler, Julia Weikum, Tine Sofie Nielsen, Annika Hult, Jens Preben Morth, Martin L. Olsson, Maher Abou Hachem; "Akkermansia muciniphila exoglycosidases target extended blood group antigens to generate ABO-universal blood"; Nature Microbiology, 2024-4-29