Nanocarrier with Escape Reflex
Tumor-specific drug release through controlled endosomal escape
Protein-based drugs must be transported into cells in a way that prevents their immediate degradation. A new approach is intended to ensure that they remain intact only in certain cells, such as cancer cells. In the journal Angewandte Chemie, a Japanese research team has introduced a nanocarrier that can “escape” from endosomes before its cargo is destroyed there. This ability to escape is only triggered within the endosomes of certain tumor cells.
© Wiley-VCH
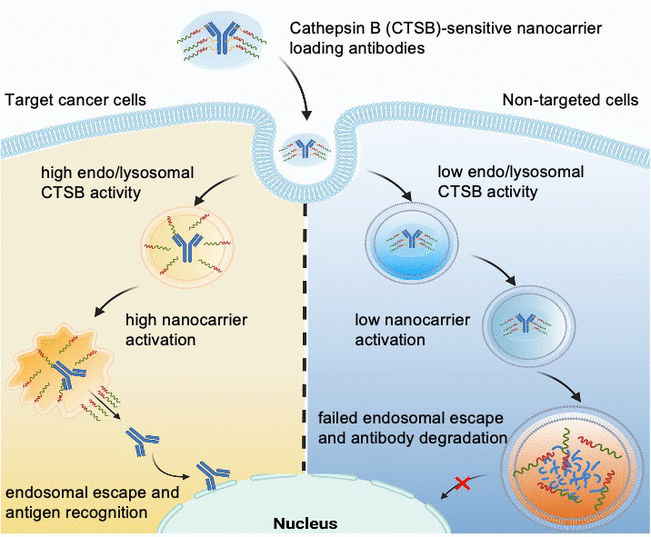
The uptake of nanocarriers into cells occurs by endocytosis: when a nanocarrier lands on the cell surface, the cell membrane folds in and encloses it in a bubble, called an endosome, which then drifts into the cell interior. In its late phase, the endosome merges with lysosomes that contain enzymes, forming an endolysosome. Within this structure, the enzymes break down both material from the body and foreign material. A protein-based drug can only become active if it “escapes” the endolysosome before being broken down. This is known as “endosomal escape”. Some nanocarriers can open the endo/lysosomal membrane and thus have endosomal escape ability.
Led by Kazunori Kataoka and Horacio Cabral, the team aims to take this a step further by producing nanocarriers for which endosomal escape is only triggered when they enter very specific cells, such as tumor cells. This would protect healthy cells. The researchers exploit the fact that different types of cells have very different endolysomomal enzyme activities. For example, the activity of the protease cathepsin B (CTSB) is especially high in cancer cells.
With the use of special fluorescence probe molecules, the team from The University of Tokyo and the Kawasaki Institute of Industrial Promotion initially studied CTSB activity and protein degradation in endosomes. They determined that in cancer cells with highly acidic endosomes, CTSB activity is already very high in their early phase—significantly before protein degradation ramps up. The researchers take advantage of this time window by using nanocarriers whose endosomal escape ability is triggered by the CTSB in cancer cells.
The team constructed poly(ethylene glycol)-based nanocarriers with diaminoethane groups capable of “tearing open” endo/lysosomal membranes. Using a linker, they then attached antibodies to act as a model for a protein drug. The nanocarrier shields the “tearing tools” so that they are initially inactive. The linker is designed to be split by the CTSB in the endolysosomes. This separates the cargo from the carrier, activating the tearing tools. They open the endo/lysosomal membrane and release intact antibodies into the cell interior—but only in tumor cells that have elevated endosomal CTSB activity.
This method could represent a new strategy for the cell-specific release of drugs through stimulus-responsive nanocarriers with controlled endosomal escape.
Original publication
Most read news
Original publication
Pengwen Chen, Wenqian Yang, Yuki Mochida, Shangwei Li, Taehun Hong, Hiroaki Kinoh, Kazunori Kataoka, Horacio Cabral; "Selective Intracellular Delivery of Antibodies in Cancer Cells with Nanocarriers Sensing Endo/Lysosomal Enzymatic Activity"; Angewandte Chemie International Edition, 2024-2-27
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Mondor's_disease
Newly developed COVID vaccine from Austria could protect against omicron and other variants - Vaccine developed at MedUni Vienna delivers promising data
National_Childbirth_Trust
American_Psychological_Association

Future Insight Prize of € 1 Million Awarded to Tobias Erb - Prizewinner from the Max Planck Institute in Marburg, Germany is working on capturing carbon dioxide and converting it into new chemical building blocks using biocatalysts
Polyneuropathy_in_dogs_and_cats
Richard_Friedrich_Johannes_Pfeiffer
Tenofovir
Purebred
Breast_cancer_chemotherapy

Making enzymes fit for industrial applications - Biobased process for ammonia production