Immune system meets cancer: Checkpoint identified to fight solid tumors
Checkpoint PHDGH in tumor-associated macrophages influences immune response and tumor growth
A study by a scientific team from the University of Vienna and the MedUni Vienna, recently published in the top-class journal Cellular & Molecular Immunology, has a promising result from tumor research: The enzyme phosphoglycerate dehydrogenase (PHDGH) acts as a metabolic checkpoint in the function of tumor-associated macrophages (TAMs) and thus on tumor growth. Targeting PHGDH to modulate the cancer-fighting immune system could be a new starting point in cancer treatment and improve the effectiveness of clinical immunotherapies.
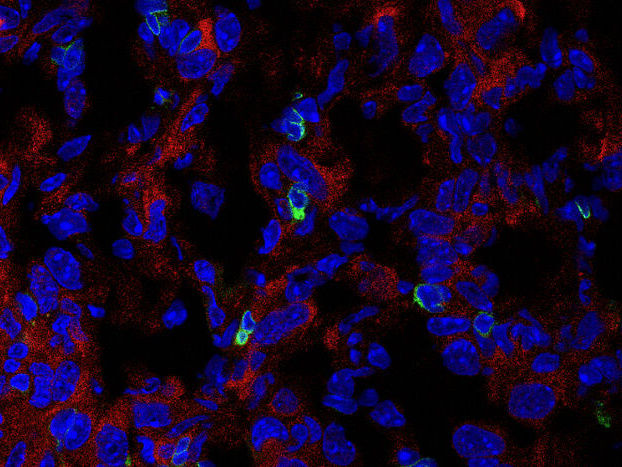
Immunofluorescence image of the expression of PHGDH (red) and CD3 T cells (green) in cryosectioned AE17 mesothelioma.
C: Zhengnan Cai
Our immune system constantly fights emerging cancer cells that arise from mutations. This process is controlled, among other things, by different types of macrophages. Tumor-associated macrophages (TAMs) are among the most abundant immune cells in the tumor microenvironment. They come from tissue-resident immune cells circulating in the blood that penetrate the tumor and differentiate there in response to various messenger substances (cytokines) and growth factors. In most solid tumors, TAMs are paradoxically considered to be tumor-promoting ("protumorigenic") overall: they promote tumor growth and metastasis by suppressing the immune response, promoting the vascular supply to the tumor and also increasing resistance to drug therapies – i.e. they generally correlate with a poor prognosis for the affected patients. Previous attempts to influence TAMs proved unsatisfactory because many patients had only a limited response to these therapeutic approaches. This underlines the urgency of finding new active ingredients and strategies.
Artificial intelligence and machine learning allow the precise diagnosis of metabolic markers and identification of checkpoints
Systems biologist and biochemist Wolfram Weckwerth from the Department of Functional and Evolutionary Ecology at the University of Vienna and last author of the study explains: "In our previous work, we have applied innovative methods of machine learning and artificial intelligence in combination with molecular analyzes to discover a new metabolic checkpoint in macrophage polarization. Our so-called ‘COVRECON strategy’ is an approach that combines various techniques from biochemistry, genetics and metabolomics with principles of mathematical control theory and dynamical systems analysis to obtain information about metabolic processes in cells. In this way, we apply fundamental concepts of artificial intelligence, which are typically used in control engineering, in biology, ecology and medicine in order to better understand processes." The researchers found that the activity of the enzyme PHDGH is controlled via different signaling pathways and in turn influences the cellular activity of the macrophages. Since TAMs resemble a subtype of normal macrophages – namely M2 – in their properties as immunosuppressive and therefore pro-tumorigenic immune cells, the scientific team set out to investigate the influence of PHDGH on their functionality and tumor growth.
Through knockout to the next round
"In the current study, together with colleagues from MedUni Vienna, we used genetic approaches to investigate the function of PHDGH in macrophages," explains study lead author Zhengnan Cai, University of Vienna. "We discovered that PHGDH is necessary for the activation of the immunosuppressive protumorigenic M2 subtype. The suppression of the PHGDH gene, on the other hand, favored the development of macrophages of the antitumorigenic type (M1) and reduced tumor growth." The research team was able to demonstrate that the PHGDH-mediated serine metabolic pathway plays a crucial role in processes associated with the regulation of the mTORC1 signaling pathway (key role in the regulation of cell growth, differentiation and cell metabolism), the activation of immunosuppressive M2 macrophages and expansion of TAMs as well as the regulation of the well-known immune checkpoint PD-L1. Wolfram Weckwerth summarizes: "Our study provides fundamental insights for understanding the interaction between immune regulation and cancer development and shows the potential to develop a strategy for modulating TAMs in the fight against tumors but needs of course further investigations."
Original publication
Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling. Zhengnan Cai, Wan Li, Sonja Hager, Jayne Louise Wilson, Leila Afjehi-Sadat, Elke H. Heiss, Thomas Weichhart, Petra Heffeter and Wolfram Weckwerth; Cellular & Molecular Immunology






















































