Curing cancer in the petri dish
Cancer researcher Dr. Christian Regenbrecht develops Reverse Clinical Engineering®
Knowing which individual cancer therapy is effective for the patient before starting treatment is the central challenge of our time. Prescribed chemotherapy fails in around half of all cancer patients. Despite treatment guidelines, neither doctors nor patients know whether a cancer drug will be effective for an individual tumor. This is because every patient reacts differently to chemotherapy. The results of a therapy can only be determined weeks or months later.
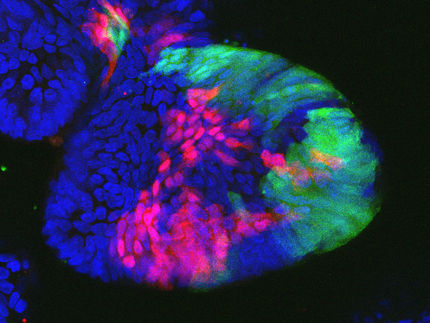
The ASC Oncology team led by cancer researcher Dr. Christian Regenbrecht has developed Reverse Clinical Engineering® test procedures to fill this gap in care: Laboratory tests are used to determine whether a drug works effectively on an individual basis. Exact copies of the patient's tumor are treated with a wide variety of cancer drugs in order to test different treatment options outside the body. Treating physicians and patients thus know which drug or drugs are likely to work or not work for the individual patient before therapy begins. For the test procedure, a fresh tissue sample is taken from the tumor to be treated during tumor surgery or a biopsy. From this tissue sample, the scientists grow 3D copies of the patient's tumor in the laboratory, so-called organoids, on which the various drugs, combinations and dosages are tested. These 3D microtumors replicate the different cell types of the tumor and are used like "crash test dummies".
The test procedure can be used to determine the optimal drug therapy for individual patients with solid tumors (carcinoma or sarcoma). A corresponding procedure for blood cancer or lymphoma is currently being optimized.
ASC Oncology was founded in 2019 by leading researchers from the fields of pathology, oncology, cell and molecular biology and biochemistry with the aim of tackling the most important challenge in modern oncology: Providing patients with the right drug at the right time.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Most read news
Other news from the department business & finance

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.