Neuromuscular 2D model for drug research
In neuromuscular diseases, the interaction between neurons and muscle cells is disrupted. Researchers led by Mina Gouti are now able to simulate this in 2D in a culture dish. The approach is well suited for high-throughput screening in drug research, they write in Nature Communications.
Around 800 different neuromuscular diseases are known today. These diseases are caused by a disturbed interaction between muscle cells, motor nerve cells and peripheral nerves. These disorders lead to muscle weakness, paralysis and in some cases death, for example in amyotrophic lateral sclerosis or spinal muscular atrophy.
"These diseases are very complex, and the cause of the disorder can lie in very different places," says Dr. Mina Gouti, head of the Stem Cell Modeling of Development and Disease research group at the Max Delbrück Center. The nerve or muscle cells, but also the connections between the two cell types, can be affected. "In order to better understand the causes and find effective therapies, we therefore need human cell culture systems in which we can investigate the interaction between spinal cord motor neurons and muscle cells."
Organoids are too large for high-throughput screening
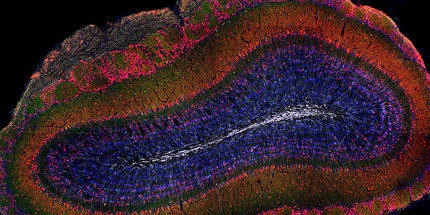
The researchers working with Gouti have already developed a three-dimensional neuromuscular organoid system, the NMOs. "One of our goals is to use our cultures to test active substances on a large scale," says Gouti. "The three-dimensional organoids are quite large and cannot be grown over a longer period of time in a 96-well culture plate, in which we can carry out high-throughput screenings with active substances."
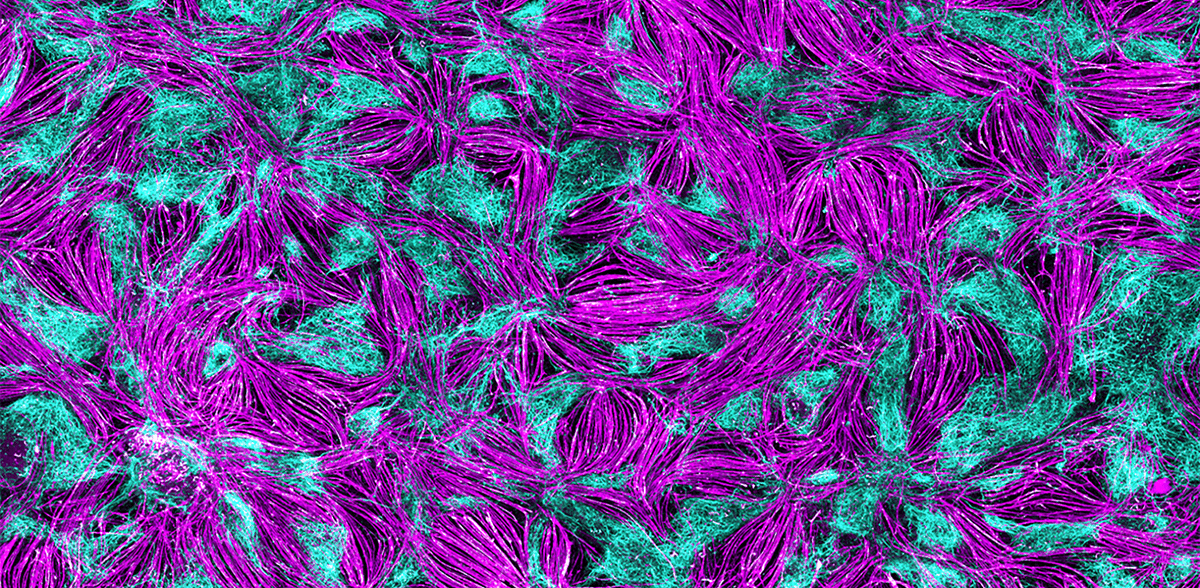
For such screenings, an international team led by Gouti has now successfully developed a self-organizing 2D cell culture system from pluripotent stem cells that contains nerve cells and muscle cells, as well as the chemical synapses, i.e. the neuromuscular junction that makes the interaction between the two cell types possible in the first place. "Using these two-dimensional cultures, we can test potential active substances in a high-throughput process as a first step and then test promising candidates in patient-specific organoids in more detail," says Gouti.
In order to establish the self-organizing 2D model for neuromuscular junctions, it was first necessary to understand how motor neurons and muscle cells develop in the embryo. Mina's team does not conduct embryo research itself, but uses various human stem cell lines that can be used for research purposes under strict guidelines, as well as an induced plutipotent stem cell line (iPSC). "We have tested various hypotheses. We realized that the cell types we need for functional neuromuscular junctions arise from neuromesodermal progenitor cells," says PhD student Alessia Urzi, first author of the publication. Urzi found the right composition of signaling molecules that help human stem cells to mature into functional motor neurons and muscle cells and to establish connections between the two. "It was very exciting to observe how the muscle cells contract under the microscope for the first time," reports the researcher. "A clear indication that we are on the right track." Another observation: the differentiated cells organize themselves into areas with muscle and nerve cells, similar to a mosaic.
An optogenetic switch for the motor neurons
The muscle cells developed in the culture dish contract spontaneously through their connection to the nerve cells, but without a meaningful rhythm. This was not enough for Urzi and Gouti. In collaboration with researchers from Charité - Universitätsmedizin Berlin, they used optogenetics to activate the motor neurons. Stimulated by a flash of light, the nerve cells fire, causing the muscle cells to contract synchronously. This brings them even closer to a physiological situation in the organism.
A model for spinal muscular atrophy
To test her model, Ursi used human iPSCs from patients with spinal muscular atrophy, a serious neuromuscular disease that occurs in children in the first year of life. The neuromuscular cultures that matured from these patient-specific pluripotent stem cells showed severe problems in the contraction of the muscle cells - similar to the muscles of the patients.
For Gouti, her 2D and 3D cultures are important tools for researching neuromuscular diseases in more detail and testing more efficient and individualized treatment concepts. In the next step, Gouti and her team want to carry out high-throughput screenings to find new treatment options for patients with spinal muscular atrophy and amyotrophic lateral sclerosis. "We first want to test whether we can achieve better treatment success for people with complex neuromuscular diseases with new combinations of active substances that have already been approved," says Gouti.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Alessia Urzi, Ines Lahmann, Lan Vi N. Nguyen, Benjamin R. Rost, Angélica García-Pérez, Noemie Lelievre, Megan E. Merritt-Garza, Han C. Phan, Gary J. Bassell, Wilfried Rossoll, Sebastian Diecke, Severine Kunz, Dietmar Schmitz, Mina Gouti; "Efficient generation of a self-organizing neuromuscular junction model from human pluripotent stem cells"; Nature Communications, Volume 14, 2023-12-19