X-ray laser uncovers the structure of a natural anti-mosquito insecticide
Bacterial protein found to work against more species of mosquito as previously thought
Advertisement
An international research team has analysed a naturally produced toxin against insects with atomic precision using the most powerful X-ray laser in the world. The data taken at the European XFEL help with figuring out the mode of action of the insecticide, which is produced by a bacterium. This information can possibly lead to the development of customised variations of the insecticide that are better tailored to specific destructive insects, such as mosquitoes that act as disease vectors. The team, led by Dominik Oberthür at DESY and Colin Berry at the University of Cardiff in the UK present their findings in the Proceedings of the National Academy of Sciences (PNAS).
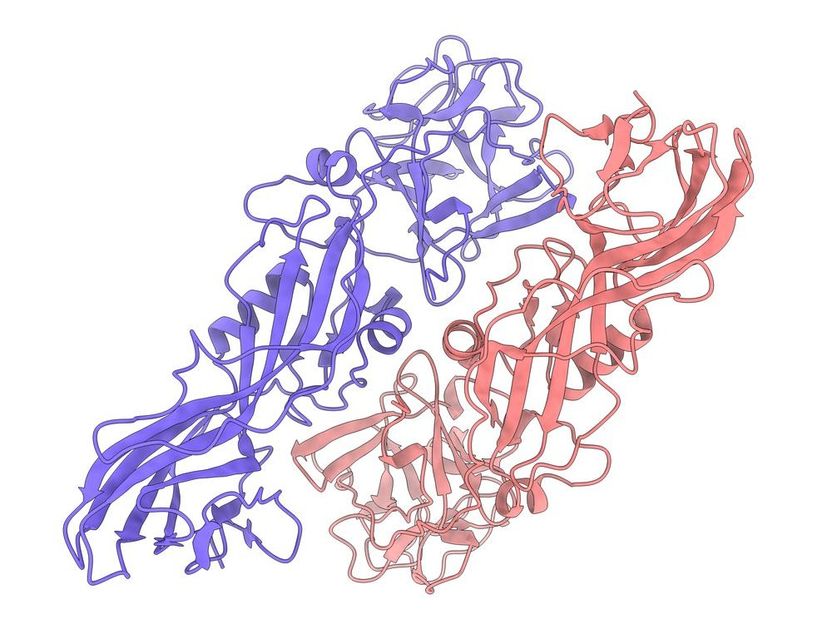
The structure of the natural insecticide protein Tpp49Aa1. The stucture was determined through analysis of the natural nanocrystals formed by the organism that generates the protein, the bacterium Lysinibacillus sphaericus.
D. Oberthür, DESY
The analysed insecticide protein is named Tpp49Aa1 and is produced by the soil bacterium Lysinibacillus sphaericus. It was known to be poisonous, in combination with another protein called Cry48, to the southern house mosquito Culex quinquefasciatus, which spreads several pathogens such as the Zika and West Nile viruses. “The insecticide actually works on more species of mosquito than we originally thought, as our investigation has shown,” reports the biochemist Berry. Collaborators in the project also tested the toxin in various species of mosquito as part of this study. They found that Tpp49Aa1, in combination Cry48, also has a toxic effect on the malaria mosquito Anopheles stephensi, the Asian tiger mosquito Aedes albopictus, and the California-native western encephalitis mosquito Culex tarsalis.
“The information about the detailed structure of the toxin reveals clues to how it might attach to the organism in order to produce its effect,” explains Oberthür. “That opens the possibility of altering it so that it binds even more specifically to an interaction site on a particular species.” That is not only interesting for the fight against disease vectors, but also for agriculture, where several bacterial toxins have been in use for a long time and a large market has grown. In both cases, the toxin should act as specifically as possible on the target pest insect and spare all other organisms.
In order to derive the structure of the protein, the researchers used tiny protein crystals that the bacterium itself produced. These crystals are typically smaller than a half of a thousandth of a millimetre. In order to illuminate this kind of crystal, extremely bright and coherent short-pulse X-ray light is necessary, which only an X-ray laser can generate. The X-ray light is scattered by the crystal, and the resulting pattern allows for the calculation of the structure of the building blocks of the crystal – in this case, the toxin proteins.
In order to get a complete picture of the protein, the crystal has to be illuminated from all sides. However, the incident X-ray laser light immediately destroys the crystal. Therefore, the researchers use a technique, called serial femtosecond crystallography, in which a plethora of crystals are analysed and the results combined into a single picture. The more data taken, the better the precision of the analysis. Thanks to the extremely high repetition rate of the European XFEL, the team could combine pictures taken from about two million tiny crystals at its SPB/SFX experiment station. The calculations produced a detailed picture of the protein at a resolution of 0.162 nanometres (a nanometre is a millionth of a millimetre), which corresponds to the diameter of a single carbon atom.
“Natural insecticides could play a key role in the fight against diseases or perhaps the protection of harvests from pests,” explains Oberthür. “With the extremely bright and intense X-ray laser pulses, we could investigate unmodified crystals of the insecticide and describe their structure. This might help in the future to elucidate how resistance against insecticides develops in insects.”



























































