Alzheimer’s Drug PRI-002 Receives EMA Approval for Phase II Trial
The Alzheimer’s drug candidate PRI-002 is entering the next clinical trial phase: the European Medicines Agency (EMA) has granted approval to conduct a phase II clinical trial. This is the first time that the drug candidate will be tested on a larger number of Alzheimer’s patients. The drug was developed at Forschungszentrum Jülich and Heinrich Heine University Düsseldorf (HHU). In September 2017, Priavoid GmbH was founded as a spin-off of Forschungszentrum Jülich with the aim of marketing the drug.
"After decades of setbacks in drug development, there have been some recent positive developments. We can't wait to start the study," explains Prof. Dieter Willbold, co-founder of Priavoid GmbH and Director at the Jülich Institute of Structural Biochemistry, as well as a Professor of Physical Biology at HHU.
The goal of the study is to demonstrate the efficacy of PRI-002 in patients at an early stage of Alzheimer's disease. For this purpose, the drug candidate will be tested in a placebo-controlled Phase II study involving 270 patients in initially six European countries. The safety of PRI-002 for healthy volunteers and patients has already been successfully demonstrated in three Phase I studies conducted in recent years.
The Phase II study, named PRImus-AD, is sponsored by the company PRInnovation in close collaboration with Priavoid and is funded by the Federal Agency for Jump Innovations (SPRIND) from the funds of the Federal Ministry of Education and Research.
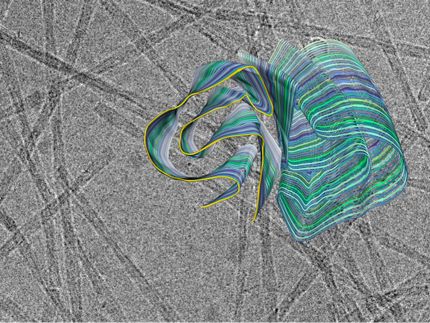
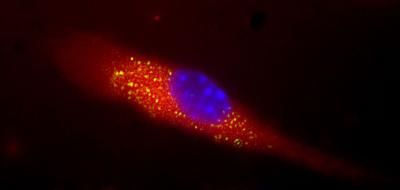
The drug candidate PRI-002 targets small soluble protein aggregates - the so-called amyloid-beta oligomers - which are considered important drivers of the disease process in Alzheimer's. PRI-002 is capable of directly dissolving these toxic amyloid aggregates into their harmless building blocks ('monomers') without the involvement of the immune system, thereby rendering them harmless. "It is, therefore, a purely physical mechanism of action," explains Dieter Willbold. "This sets PRI-002 apart fundamentally from drug candidates based on antibodies, which are currently seeking drug approval."
Great hopes are pinned on the now-approved Phase II study, as Germany alone has approximately 1.1 million Alzheimer's patients for whom, to date, no effective, disease-modifying, and safe medications have been approved.
The results of this study are expected in 2026. The aim is to seamlessly transition into a corresponding Phase III approval study thereafter.
Most read news
Topics
Organizations
Other news from the department research and development

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.