Automated, cost-effective production of mRNA vaccines as well as cell and gene therapeutics
“The system will enable automated, component-based, flexible and validatable production”
mRNA-based vaccines and gene/cell therapeutics open up new possibilities for medical practitioners in the fight against cancer and infectious or hereditary diseases. However, manufacturing these innovative pharmaceuticals is an expensive and time-consuming process. The Fraunhofer RNAuto lighthouse project is aiming to use automated production technologies to facilitate cost-effective, high-volume production of mRNA-based drugs and medicines for personalized therapies which can then be made available at an affordable price. From November 13 to 16, researchers from the Fraunhofer-Gesellschaft will, for the first time, present a screening system demonstrator for the automated encapsulation of mRNA in nanotransporters at COMPAMED in Düsseldorf (Hall 8a, Booth G10).
Automated production technologies for mRNA-based vaccines as well as cell and gene therapeutics.
© Fraunhofer IZI
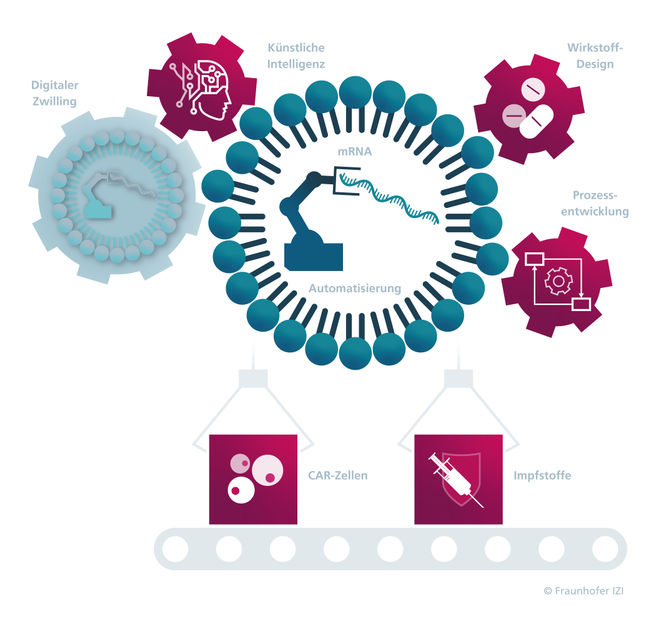
The development of mRNA vaccines and pharmaceuticals for innovative therapies such as gene and cell therapeutics is booming. Driven in part by the COVID-19 pandemic, the market for advanced therapy medicinal products (ATMPs) is growing steadily. These pharmaceuticals for innovative therapies represent a groundbreaking milestone in the treatment of complex, previously incurable diseases, including many types of cancer. However, ATMPs are still only administered in rare cases — due to the inconvenience of the laborious, cost-intensive manufacturing process which, up to now, has often involved making the products manually. To provide as many patients as possible with customized, personalized therapies, the production processes will need to be digitalized and automated. The seven Fraunhofer institutes involved in the RNAuto lighthouse project are therefore pooling their expertise from the fields of medicine, biology and engineering in order to facilitate the fast, automated and cost-effective production of large quantities of mRNA vaccines as well as gene and cell therapeutics that use mRNA as a starting material. One area that the researchers are focusing on is the development of a screening system — capable of being scaled up to industrial level — with digital process management and data-driven quality control, which can be used to encapsulate mRNA (messenger RNA) in lipid nanotransporters. As a first step, the project team is aiming to produce mRNA active ingredients on a laboratory scale in quantities up to 20 ml. In collaboration with the Fraunhofer Institutes for Microengineering and Microsystems IMM, for Production Technology IPT and for Cell Therapy and Immunology IZI, the Fraunhofer Institute for Experimental Software Engineering IESE is coordinating the design and development of the automated, component-based screening system.
Replaceable, combinable components
The system measures 1.20 m x 0.90 m x 0.90 m and is made up of scalable, flexible and non-proprietary production modules, which can be replaced via a plug-and-play system in the event of a defect.
In order to produce a reliable mRNA pharmaceutical, consistent product quality must be guaranteed. This includes quantifying the amount of encapsulated mRNA in the nanotransporters. The inconsistent ratio of encapsulated mRNA at the start of the mixing process and the high throughput rates in the production process pose challenges for continuous quality control. “The technique for encapsulating mRNA in the lipid capsules/pellets is extremely complex. Numerous parameters — such as the length and structure of the mRNA, the size, viscosity and charge of the lipids, or machine settings such as pressure, flow speeds and temperature — have an impact on the quality of encapsulation,” says Rolf Hendrik van Lengen, Program Manager Digital Healthcare at Fraunhofer IESE. Due to the resulting variation in the viscosities of the mixture, refractive indices, conductivities, temperatures and pH values and the corresponding impact on product quality, process optimization is crucial along with comprehensive quality control for the fractions.
System with integrated online analytics for digital quality assurance and documentation
All aspects of quality assurance and process control (i.e., control of the pump, mixer, etc.) are mapped digitally via digital twins and AI-based software tools — a first in the production of mRNA active ingredients. To do this, the researchers are harnessing their experience from Industry 4.0 and are using software such as the Eclipse BaSyx Industry 4.0 middleware from Fraunhofer IESE and the COPE process control software from Fraunhofer IPT. The required digital information is delivered to the digital twins via dynamic light scattering (DLS) — a measurement technique for characterizing particle sizes in emulsions — as well as additional temperature and pressure sensors so it can be documented, with each component represented by its own digital twin.
The screening system allows the project partners to find the ideal combination of mRNA and lipids and the best possible degree of encapsulation. Each individual attempt with modified parameters can be logged digitally using the digital twin for product quality. This means that any errors — such as excessively low temperatures — can be documented and made available digitally. Once the system reaches the final expansion stage, the digital twin will be able to initiate appropriate corrective measures when a situation like this occurs. “With our integrated online analytics, we can measure quality automatically during the manufacturing process in order to determine the ideal composition of the active ingredient. For many pharmaceutical manufacturing processes, it is standard practice to take samples during the process and have them analyzed externally. In the worst-case scenario, entire batches have to be discarded. We can avoid this with our online analytics,” explains computer scientist van Lengen. “The system will enable automated, component-based, flexible and validatable production.” Once the system is complete, the research team will be able to produce 20 ml of quality-assured mRNA active ingredient within a few seconds.
As soon as the system is ready, the mRNA active ingredient will be produced automatically in the RNAuto project as a prophylaxis against the West Nile virus, and its efficacy will be tested at Fraunhofer IZI in Leipzig. The completed system is expected to be ready for use at Fraunhofer IMM in Mainz by the end of 2025 and will also be made available to industry partners. The key parts of the system demonstrator — the pumps, the mixer and the digital components — will be showcased at the joint Fraunhofer booth (Hall 8a, Booth G10) at COMPAMED in Düsseldorf from November 13 to 16.
See the theme worlds for related content
Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.

Topic world Gene therapy
Genetic diseases once considered untreatable are now at the center of innovative therapeutic approaches. Research and development of gene therapies in biotech and pharma aim to directly correct or replace defective or missing genes to combat disease at the molecular level. This revolutionary approach promises not only to treat symptoms, but to eliminate the cause of the disease itself.