Small protein, big impact
A small protein acts as a sensitive alarm for bacteria to be alert to antimicrobial peptides
Nature uses antimicrobial peptides as broad-spectrum antibiotics: They form the first line of defense against invading pathogens. bacteria, in turn, have developed ways to circumvent these defenses. Microbiologists at the Max Planck Institute in Marburg have investigated how a small protein enables bacteria to sensitively recognize antimicrobial peptides and effectively defend themselves. Their study provides the molecular basis for the development of new peptide-based agents.
Small proteins are typically less than 50 amino acids in length. Because of their small size, they have been largely overlooked in the past. Advances in bioinformatics and ribosomal profiling have revealed that hundreds to thousands of small proteins are produced in bacteria, archaea, eukaryotes and microbial communities. Studies have shown that they function primarily as regulators of various biological processes and pathways.
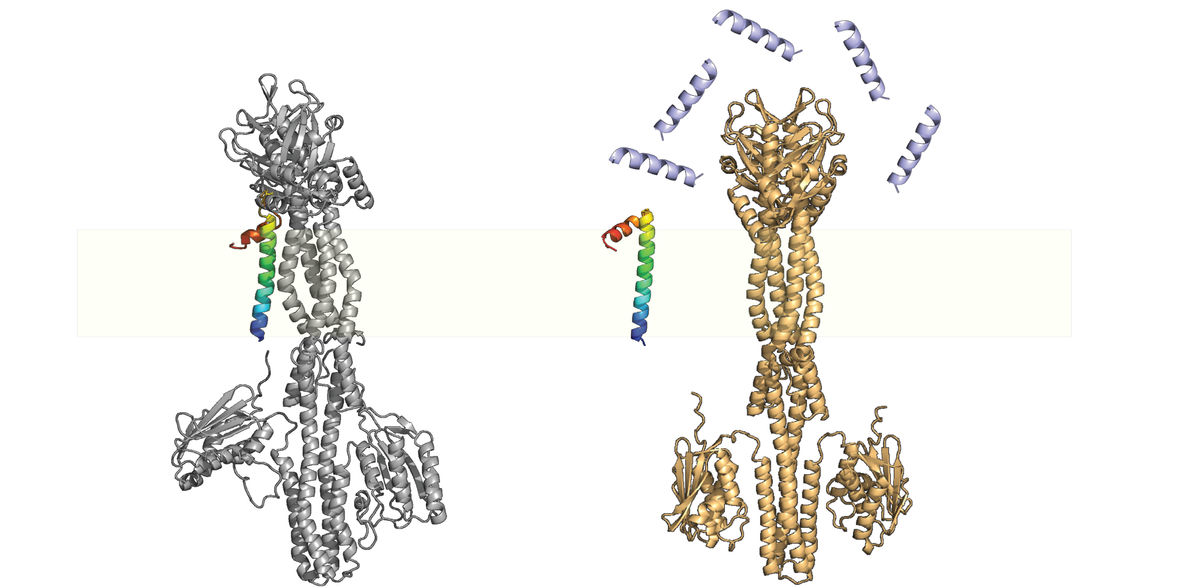
Project group leader Dr. Jing Yuan of the Max Planck Institute for Terrestrial Microbiology calls small proteins "nature's gadgets." Among other things, her team is studying an important small protein called MgrB. It is widely distributed in enterobacteria and interacts with a sensor kinase called PhoQ.
The sensor kinase is a membrane bound protein that senses environmental cues associated with the host, such as magnesium limitation, decreased pH, increased osmolarity, long-chain unsaturated fatty acids from bile, and antimicrobial peptides. With a properly functioning PhoQ/PhoP system, pathogenic bacteria can activate the virulence program and evade the immune system inside the mammalian host at the right time and place. The two-component PhoQ/PhoP system is thus a master regulator of the virulence program, and MgrB's interaction affects bacterial virulence, fitness, and drug resistance. But how exactly?
When bacteria invade the host, the PhoQ/PhoP system is activated due to the relatively low physiological concentration of magnesium, which then induces the expression of mgrB, resulting in a partially repressed PhoQ/PhoP system. In the presence of antimicrobial peptides (e.g. in the intestinal mucus layer or inside macrophages), MgrB dissociates from PhoQ, releases the inhibitory effect and triggers the full activation of the PhoQ/PhoP pathway.
The research team, together with colleagues from the University of Bern, Switzerland, has now shown that MgrB's inhibition of PhoQ stabilizes the system by providing negative feedback. Without MgrB, the PhoQ/PhoP system is hyperactive and no longer sensitive to subinhibitory levels of antimicrobial peptides.
"It is like a person throwing fists in the dark, not knowing if it is safe or if there`s an attack," says Jing Yuan. “Our results demonstrate how important such a small protein can be for bacterial fitness and drug resistance “Moreover, it also provides the molecular basis for engineering novel peptide-based regulators to control bacterial virulence and drug resistance.”
By tweaking the properties of MgrB, the Yuan group is currently developing novel peptide regulators they call Super MgrBs. Super-MgrBs are expected to bind permanently to PhoQ, thereby inhibiting it. This would completely suppress the PhoQ/PhoP system and keep the bacterial defense program in an off-state. The researchers hypothesize that super-MgrBs could prevent the recognition of antimicrobial peptides, making pathogenic bacteria more vulnerable.