New study on the genetic magnetization of living bacteria
Great potential for biomedicine
Magnetic bacteria possess extraordinary capabilities due to the magnetic nanoparticles, the magnetosomes, which are concatenated inside their cells. A research team at the University of Bayreuth has now transferred all the about 30 genes responsible for the production of these particles to non-magnetic bacteria in a broad series of experiments. This resulted in a number of new bacterial strains that are now capable of producing magnetosomes. The research findings presented in "Nature Nanotechnology" are groundbreaking for the generation of magnetized living cells, which have great potential for the development of innovative diagnostic and therapeutic methods in biomedicine.
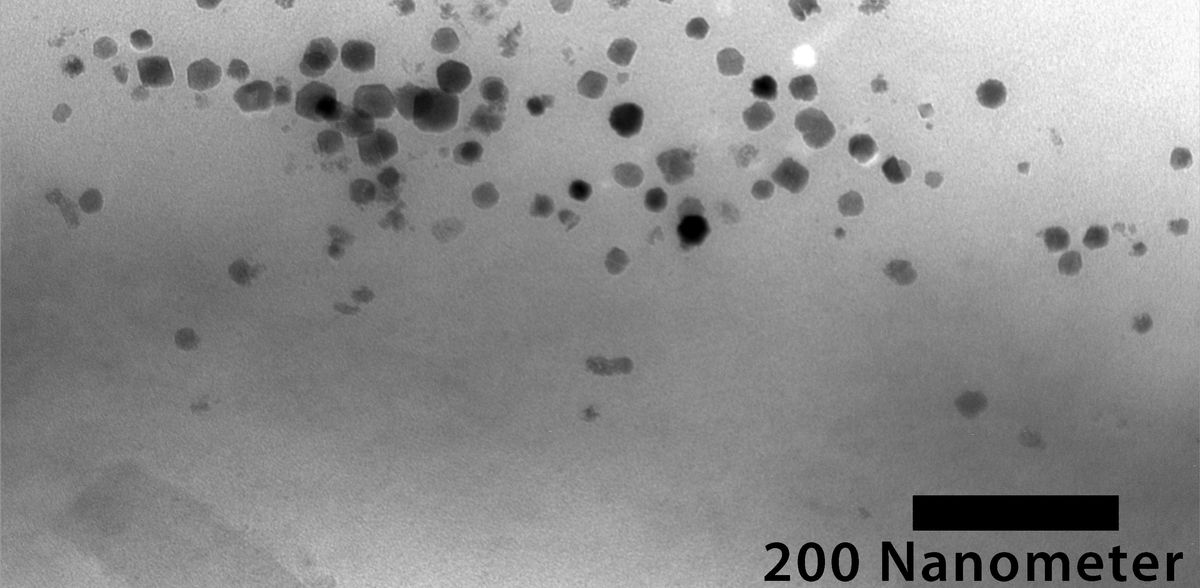
Based on extensive studies, the researchers initially identified 25 species of non-magnetic proteobacteria – by far the most extensive domain of bacteria – that are particularly suitable for gene transfer and for studying magnetosome formation. Both biochemical properties and the availability of specific gene sequences were decisive factors. Magnetization was successful in seven species: these bacteria continuously produce magnetosomes in which iron-containing magnetite crystals are chained together in a manner similar to that in the donor bacterium Magnetospirillum gryphiswaldense.
"In terms of future applications in biomedicine, it is particularly promising that two species of bacteria that we have successfully genetically engineered are already widely used in biotechnology. According to the current state of research, they are well compatible with human cells. This opens up new perspectives for a variety of biomedical applications – for example, for microrobot-controlled transport of active pharmaceutical ingredients, for magnetic imaging techniques, or even for optimizations of hyperthermia cancer therapy," says the first author of the new study, Dr. Marina Dziuba, who is a research associate at the Microbiology research group in Bayreuth.
The Bayreuth researchers have studied the magnetosomes produced by the new transgenic bacterial strains in more detail and thus identified a number of factors that could be causally involved in magnetosome formation. Comparison between the genome of these strains and the genome of those genetically modified bacteria that failed to produce magnetosomes has also led to valuable insights. There is much evidence to suggest that the magnetosome formation of transgenic bacterial strains is closely related to their ability to photosynthesize or to engage in oxygen-independent, so-called anaerobic respiration processes. Overall, the new study shows that it is not single or a few particular genes that transgenic bacteria lack when they are incapable of magnetosome formation. Rather, the decisive factor for them to synthesize magnetosomes after receiving the foreign gene clusters is a combination of certain metabolic properties and the ability to efficiently use the genetic information of the foreign genes to produce cellular proteins.
"Our study shows that further research is needed to understand the biosynthesis of magnetosomes in detail, identify barriers to their transfer, and develop strategies to overcome them. At the same time, however, our results shed new light on metabolic processes that support magnetosome formation. They therefore provide a framework for future investigations on the way to designing new strains of biocompatible magnetic bacteria tailored for biomedical and biotechnological innovations," explains Prof. Dr. Dirk Schüler, Chair of Microbiology at the University of Bayreuth.
In earlier research, the Bayreuth team had already succeeded in introducing the genes responsible for magnetosome formation from the bacterium Magnetospirillum gryphiswaldense – a model organism for research – into the genome of non-magnetic bacteria. However, in only a few cases, this gene transfer resulted in genetically modified bacteria that, in turn, began to form magnetosomes. It remained completely unclear which factors might influence whether transgenic bacteria produced magnetosomes. Against this background, the study now published, in which a research partner at the University of Pannonia in Veszprém/Hungary also participated, provides important new impetus for the targeted magnetization of living cells.