Reducing the carbon footprint of methane by converting it into methanol with a new enzyme
Advertisement
A team led by Professor Osami Shoji at Nagoya University in Japan has developed a technology to convert methane, the principal component of natural gas, into Methanol at room temperature in water. They used an enzyme that can be easily mass-produced, offering the possibility of a cheap and effective means to reduce the carbon footprint of natural gas. They published the results in ACS Catalysis.
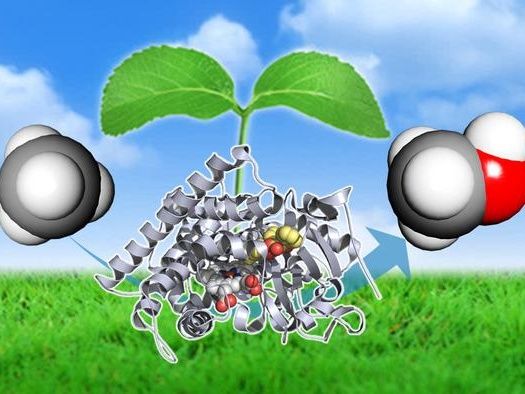
New technology converts a component of natural gas, methane (left), to methanol (right), using the P450BM3 enzyme (middle, grey) with a decoy molecule (middle, colored). This method can be a cheap and effective mean of reducing the carbon footprint of natural gas.
Ariyasu Shinya
Methane is the key component of natural gas and an abundant natural resource. However, it is chemically stable, requiring huge amounts of energy before it undergoes chemical conversion. One solution is to convert methane to methanol. Methane can be converted to methanol, which is cleaner than other fossil fuels and can be easily stored and transported. Converting methane to methanol can be done using the methane monooxygenase enzyme. However, the enzyme has a complex structure, making it difficult to handle and unsuitable for mass production.
Enzymes are usually very specific, often compared to a key for a particular lock. Converting methane to methanol using enzymes other than methane monooxygenase was thought to be impossible. However, the research group turned to their previous work on the addition of chemically synthesized molecules to an enzyme to change the characteristics of the enzyme itself. This enables the chemical conversion of compounds that would not normally be accepted, a process called a substrate misrecognition system.
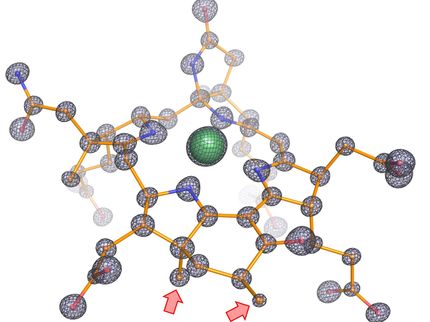
“In this system, artificial molecules called decoy molecules are designed and synthesized to resemble the compounds, called substrates, that the target enzyme usually accepts”, said Shoji. “When added to the enzyme, the enzyme mistakenly takes in the "decoy molecules" as the original target compound, and the enzyme is activated. If a molecule, in this case methane, that is normally unreactive, is added to the enzyme, the enzyme will mistake the decoy molecule for the original target compound and take it in. The activated enzyme then converts the methane to another molecule by ‘mistake’.”
The research group used chemical enzyme control technology on the enzyme P450BM3. It hydroxylates long-chain fatty acid molecules and has been used to convert similar substances such as benzene, ethane, and propane. However, as these substances are more reactive and larger than methane, methane conversion presented a greater challenge.
The group next searched for decoy molecules with an optimal structure to anchor the smallest methane molecule in the reaction pocket of P450BM3. The researchers investigated about 40 molecules that had been found to be effective in ethane hydroxylation from a library of about 600 decoy molecules. In a breakthrough, Shoji confirmed that the most efficient decoy molecule could convert methane to methanol in water at room temperature.
“When we tested it, we successfully converted methane to methanol using P450BM3,” said Shoji. “This was an exciting breakthrough as P450BM3 is derived from the bacterium Priestia megaterium (formerly Bacillus megaterium), making it is easy to handle and produce in large quantities using E. coli. This makes it an attractive new option for the effective utilization of methane gas.”
One day, decoy molecules could enable the conversion of compounds less difficult than methane. “We expect that the technology can be developed into a low-energy, environmentally friendly conversion technology for many other hydrocarbons besides methane,” said Shoji. “Therefore, it is expected to contribute to the promotion of the use of enzymes in the discovery of low environmental impact substance conversion technologies in Japan. We expect this achievement to have a significant impact on the fields of catalytic and enzymatic chemistry.”
Japan may prove to be an ideal test site for their technology because of discoveries of large amounts of methane buried as methane hydrate in the surrounding seas. Shoji is optimistic about the potential for using this untapped resource. He said, “Developing effective methods of methane utilization is an important issue for both solving environmental problems and increasing the efficiency of resource use. We hope that our research can help to solve the problem of limited natural resources in Japan.”
Original publication
Shinya Ariyasu, Kai Yonemura, Chie Kasai, Yuichiro Aiba, Hiroki Onoda, Yuma Shisaka, Hiroshi Sugimoto, Takehiko Tosha, Minoru Kubo, Takashi Kamachi, Kazunari Yoshizawa, Osami Shoji; "Catalytic Oxidation of Methane by Wild-Type Cytochrome P450BM3 with Chemically Evolved Decoy Molecules"; ACS Catalysis, Volume 13, 2023-6-14