Repellant and Hostile to Bacteria
Zwitterionic polymeric sulfur ylides: antifouling coating with a synergistic effect
Advertisement
bacteria that gather into biofilms on the surfaces of implants, catheters, breathing tubes, and other medical components are a serious health hazard. In the journal Angewandte Chemie, a research team from the Netherlands has now introduced a new material based on poly(sulfur ylides) that—when applied as coating—effectively inhibits this process known as fouling. The coating minimizes the adhesion of bacteria to surfaces and is also a bactericide while not affecting mammalian cells.
Bacteria organized into biofilms are especially stubborn and often resistant to antibiotics. It is estimated that 65% of infections acquired in hospitals originate from biofilms. The cause is frequently contamination with infectious bacteria from a patient’s skin or pathogens that circulate in the bloodstream. The first step is adhesion of the bacteria to a surface. To inhibit this, exposed surfaces are given antifouling coatings, usually made of polyethylene glycol (PEG). PEG binds to water molecules, which then form a hydration layer—an effective barrier against the undesired adsorption of biomolecules and bacterial cells. However, recent research has revealed that PEG also has disadvantages in that it seems to trigger immune responses.
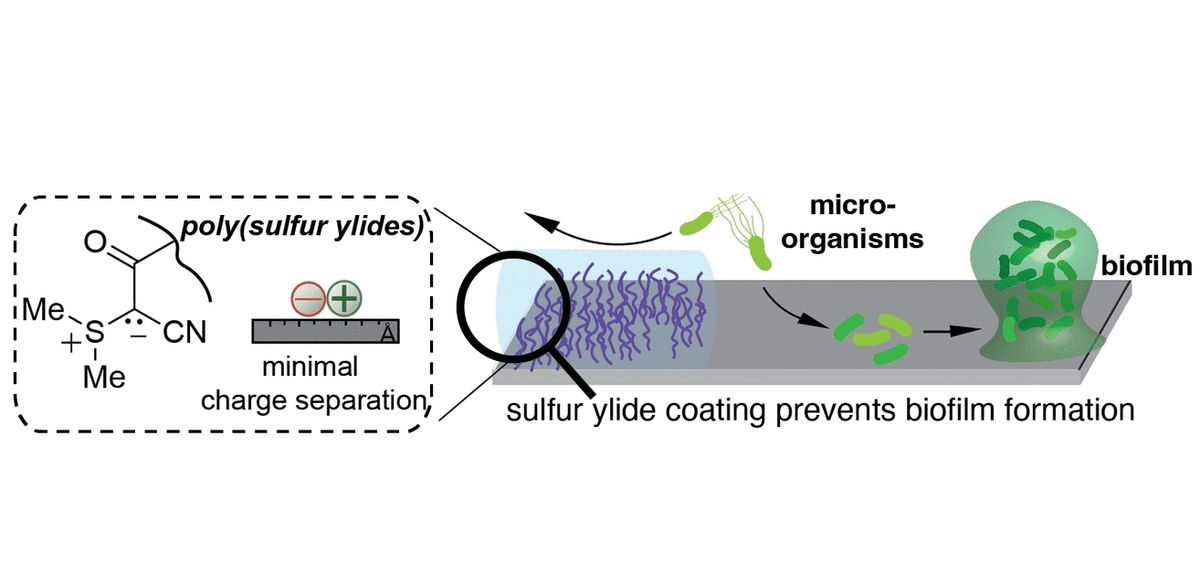
An alternative solution is offered by polybetaines, a class of zwitterionic polymers. A zwitterion is a molecule that carries both positive and negative charges. Recent studies suggest that the antifouling performance enhances as the distance between the positive and negative charges grows smaller. The most effective of these substances should have the negatively charged atoms directly adjacent to the positively charged ones—a requirement that cannot be met with a conventional betaine structure. However, this is precisely the unique structural feature of ylides. For example, in a sulfur ylide, a positively charged sulfur ion is bound directly to a negatively charged carbon atom.
A team led by Daniela A. Wilson and Kevin Neumann at Radboud University in Nijmegen (Netherlands) produced zwitterionic polymers based on sulfur ylides, poly(sulfur ylides) abbreviated as P(SY), which display sulfur ylides as side chains on a backbone made of polystyrene. The team showed that the new P(SY) had antifouling properties exceeding those of PEG. This seems to be the result of a synergistic effect. Like PEG, P(SY) produces a hydration layer that inhibits the adhesion of bacteria and biomolecules. Unlike PEG, P(SY) also reduces the viability of bacteria that overcome the barrier of the hydration layer. Presumably, these initially bind to the ylide groups through electrostatic attractions. Once they come into contact with the hydrophobic polystyrene backbone, their cell membranes become porous, and the bacterial cells die off. Mammalian cells, in contrast, are not affected by P(SY); in fact, fibroblast cells, a type of connective tissue, were grown on poly(sulfur ylide) coatings.