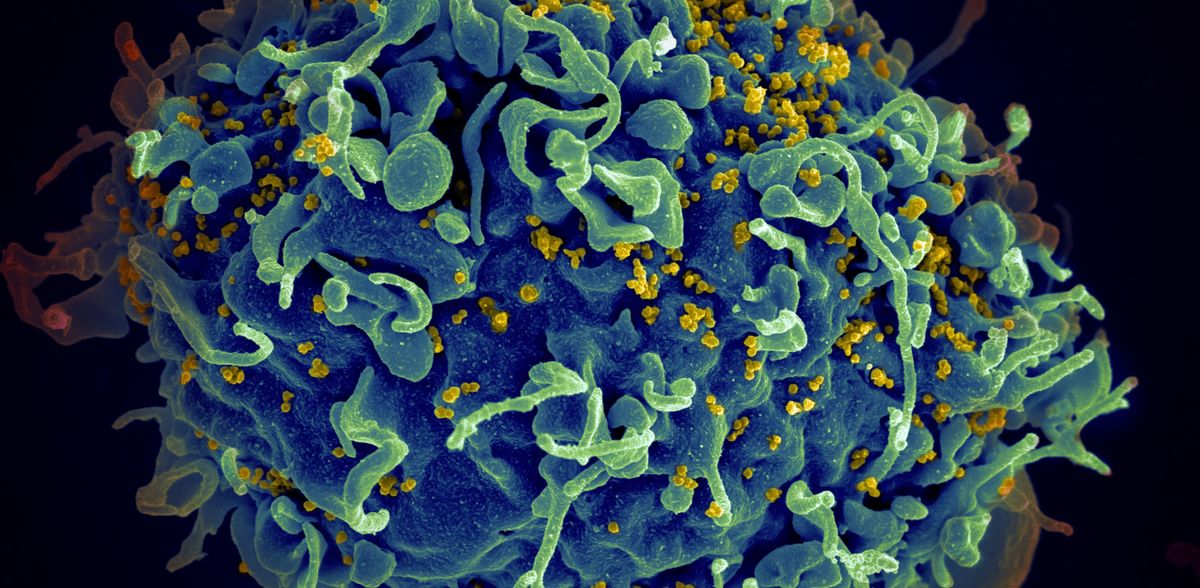
Researchers Find New Pathway for HIV Invasion of Cell Nucleus
A study has identified a new pathway that human immune deficiency virus (HIV) uses to enter the nucleus of a healthy cell, where it can then replicate and go on to invade other cells.
The researchers also identified three proteins that are needed for the virus to carry out the invasion and have in turn synthesized molecules (potential drugs) that can target one of the proteins, potentially leading to new treatments for AIDS.
“We have revealed a protein pathway that appears to have a direct impact on diseases, which opens up a new area for potential drug development,” says the study’s senior author Aurelio Lorico, MD PhD, Professor of Pathology and interim Chief Research Officer at Touro University Nevada College of Osteopathic Medicine.
HIV infection requires the virus to enter a cell and gain access to the well-guarded nucleus in order for the viral components to be integrated into the healthy cell’s DNA. But how the viruses get past the protective membrane is not well understood and is the subject of much debate.
The newly identified pathway begins with HIV entering a cell wrapped inside a membrane package, called an endosome. The virus-containing endosome then pushes the protective nuclear membrane inward, forming an indentation known as a nuclear invagination. The endosome then moves inside the invagination to its inner tip, where the virus then slips into the nucleus.
The study found that three proteins were critical to the invasion: One protein (Rab7) is located on the membrane of the endosome, the second (VAP-A) is on the nuclear membrane where the invagination occurs, and the third (ORP3) connects the first two proteins together. An interaction among the three proteins is needed for the invasion to be successful, so targeting any of these proteins could halt the infection. The team has synthesized and tested molecules that interrupt the interaction among the proteins. The researchers observed that, in the presence of these molecules, HIV replication does not occur.
This pathway for nuclear access was first discovered in the team’s research on cancer metastasis and is likely involved in other diseases as well.
“This is an entirely new pathway and we have developed molecules (drugs) that block it,” says Lorico. “Although our research is at a pre-clinical stage, it is likely that the new drugs synthesized may have therapeutic activity in AIDS, other viral diseases, and possibly metastatic cancer and other diseases where nuclear transport is involved.” The team is currently looking at the pathway’s role in Alzheimer’s disease and metastasis of many types of cancer.
“Because the pathway we found may apply to many types of disease, there is a tremendous amount of work that needs to be done to understand the full benefits of this research,” says Dr. Denis Corbeil, co-leading author of the study, research group leader at the Biotechnology Center (BIOTEC) of TUD Dresden University of Technology in Germany.
“The ground-breaking research of Dr. Lorico and his team is a testimony to the importance that Touro University gives to its mission of service to humanity. The potential therapeutic applications of this new pathway to improve patient care are immense and may help us better navigate the next pandemic,” said Dr. Alan Kadish, Touro University President.
The study, HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells, was the result of a collaboration of researchers from Touro University Nevada College of Osteopathic Medicine, Touro College of Osteopathic Medicine in New York, researchers from the Biotechnology Center (BIOTEC) of TU Dresden University of Technology in Germany, and researchers from Italy.
Original publication
Mark F. Santos, Germana Rappa, Jana Karbanová, Patrizia Diana, Girolamo Cirrincione, Daniela Carbone, David Manna, Feryal Aalam, David Wang, Cheryl Vanier, Denis Corbeil and Aurelio Lorico: "HIV-1-induced nuclear invaginations mediated by VAP-A, ORP3, and Rab7 complex explain infection of activated T cells."; Nature Communications (August 2023)