Antibiotic ribosomal compounds deciphered
Combating multidrug-resistant bacteria through high-resolution structural imaging
A European research team led by the Department of Chemistry at the University of Hamburg presents structures of 17 different antibiotic ribosomal compounds with high detail resolution in a study. The knowledge could pave the way for the development of novel antibiotics to combat multidrug-resistant bacteria.
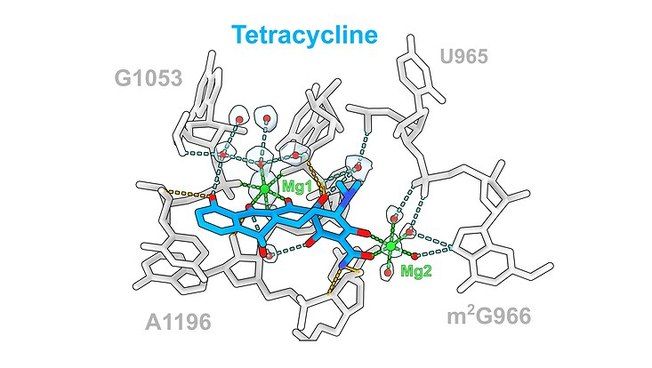
Antibiotic tetracycline (blue) and its interactions with ribosomal rRNA Photo: UHH/Paternoga The antibiotic tetracycline (blue) and its interactions with ribosomal rRNA (gray), which are mediated via direct (orange) or indirect (cyan) interactions via Mg1 (green) or water molecules (red).
UHH/Paternoga
Ribosomes are large molecular complexes found in the cells of plants, animals, humans as well as bacteria that produce proteins. Therefore, they are important targets for antibiotics, which in bacteria couple to a subunit of the ribosomes and cause reading errors or even reading stops. This results in the formation of defective proteins that lose their biological function, and the bacterium dies. However, more and more multi-resistant bacteria can prevent antibiotics from working.
To circumvent these resistances, a detailed understanding of antibiotics is essential, especially antibiotic ribosome structures. Over the past two decades, antibiotic ribosome structures for each major class of ribosome-directed antibiotics have been published, providing insights into their binding sites and mechanisms of action.
The team led by Prof. Daniel N. Wilson, Ph.D., of the Department of Chemistry at the University of Hamburg, Germany, has now used cryo-electron microscopy to image these structures at a resolution of 1.6 to 2.2 Ångstroms (Å), where one Å corresponds to the ten-millionth part of a millimeter. "In the past, antibiotic ribosome structures were mainly presented at resolutions of 2.5 to 3.5 Å," said Prof. Daniel N. Wilson, PhD. "The improved resolution now allows us to observe even water molecules that support drug-drug interactions." The researchers found that generally ten to 20 hydrogen bonds interacted with the ribosome, with the contribution of water molecules varying for different classes of antibiotics.
The antibiotics studied included the six clinically relevant antibiotic families, including the tetracyclines used for respiratory infections, aminoglycosides with a broad spectrum of activity, including tuberculosis or meningitis, and the pleuromutilins for skin and soft tissue infections.
For the study, the research team applied these antibiotics to so-called 70S ribosomes of the bacterium "Escherichia coli" - a coliform bacterium that is also found in the human intestine. "We anticipate that this information can be used in the future for structure-based design of new antibiotic derivatives. By identifying regions that can be altered, the bound water can be selectively displaced to take on an interaction with the target, which is the bacterium," Wilson says.
The high-resolution imaging is a big step, according to Wilson: "The old antibiotic ribosome structures at lower resolution show that the binding sites for each class of antibiotic are similar, but in many cases there are profound differences in terms of the exact location of the drugs as well as the drug-binding site." Therefore, he said, the high-resolution experimental data were needed to provide a more accurate description of the interactions of antibiotics with the ribosome.
Note: This article has been translated using a computer system without human intervention. LUMITOS offers these automatic translations to present a wider range of current news. Since this article has been translated with automatic translation, it is possible that it contains errors in vocabulary, syntax or grammar. The original article in German can be found here.
Original publication
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Last viewed contents
Illumina and Oxford Nanopore Enter into Broad Commercialization Agreement - Single-Molecule DNA Sequencing System Promises Dramatic Improvement in Cost, Speed, Versatility, and Simplicity of Sequencing

Casting off: Leaving autoimmune disease behind thanks to an innovative treatment - Research team makes decisive breakthrough
APEIRON Biologics announces financing round for the further development of the COVID-19 drug APN01 - Financing secures further clinical trials and the supply of material for the treatment of COVID-19 patients
NovogeneAIT Singapore and the Genome Institute of Singapore forge Public-Private Partnership - Establishing whole genome sequencing centre in Singapore
Gene that influences the ability to remember faces
Estación_de_Fotobiología_Playa_Unión
E. coli bacteria's defense secret revealed
Felicitex Therapeutics and Selvita Initiate Strategic Collaboration to Target Cancer Quiescence

Faecal Pollution: DNA Uncovers Culprit - CSI and forensics can be used to uncover not only serial killers but also the cause of water pollution
Chinese_Mental_Health_Association
Catalent Invests $7.3 Million in Italy - Expands Softgel Encapsulation and Packaging Capabilities in Support of Consumer Health






















































