New muscle therapy gets fast-track boost
Berlin start-up could soon be helping children with previously incurable muscle diseases thanks to an accelerated approval process
To help bring therapies for rare muscle diseases in children to market sooner, the Berlin-based start-up MyoPax, a spin-off from the Max Delbrück Center and Charité – Universitätsmedizin Berlin, has now received a boost from the U.S. Food and Drug Administration (FDA). The company has been granted the FDA’s orphan drug designation (ODD) and rare pediatric disease designation (RPDD), both of which offer multiple regulatory and financial advantages – including fast-track approval status and, eventually, market exclusivity.
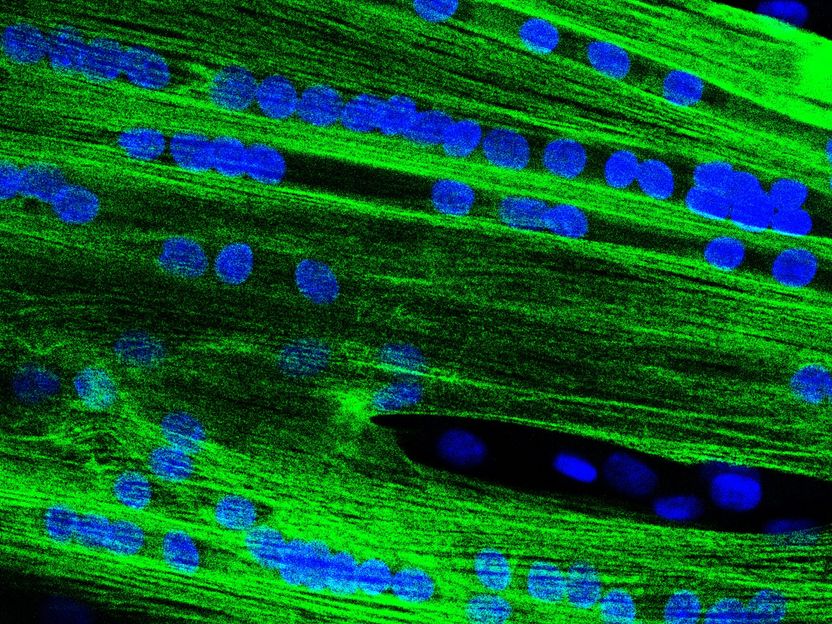
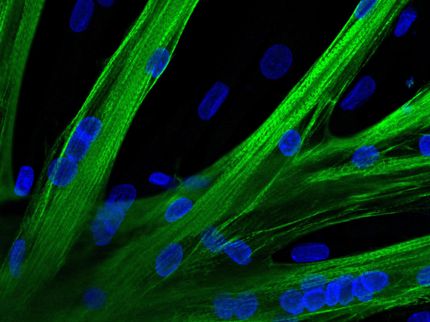
Individual muscle stem cells fuse to form multinucleated elongated muscle fibre precursors.
Dr. Eric Metzler, Wissenschaftler der MyoPax GmbH
But first, the researchers must test their new therapeutic approach in a clinical trial sponsored by Charité. The German Federal Ministry of Education and Research and ForTra gGmbH of the Else Kröner-Fresenius Foundation are funding the trial, which will start with the first patients in the fall and should be completed by 2026.
The result of many years of research
The innovative muscle stem cell technology selected by the FDA is based on years of research by Professor Simone Spuler and her Myology Lab team at the Experimental and Clinical Research Center (ECRC), a joint institution of the Max Delbrück Center and Charité. Spuler founded MyoPax last year, together with Dr. Verena Schöwel-Wolf. Their goal is to develop therapies for local muscle defects, acute muscle wasting and hereditary muscular dystrophies that are currently incurable or for which adequate treatment does not yet exist.
With their stem cell therapy, the team wants to help children who suffer from exstrophy-epispadias complex (EEC). This spectrum of rare congenital disorders is characterized by the usually hollow bladder lying inverted and opened like a plate on the abdominal wall. In addition to malformations of the abdominal muscles, pelvic bones, urethra and external genitalia, the bladder sphincter also remains underdeveloped. This muscle defect is due to delayed cell migration during embryonic development and causes lifelong incontinence. About one in 11,000 children is born with EEC and the malformations are currently surgically corrected. Often, further surgery is required to improve bladder function. With the help of MyoPax’s new stem cell therapy, however, bladder sphincter muscle can be rebuilt, thus allowing the patient to regain long-term bladder control.
“Receiving this recognition from the FDA is an important milestone in our work,” says Spuler. “It confirms that our new therapeutic approach has the potential to improve the lives of young patients with EEC and other muscle diseases – as well as the lives of their parents.”
With her second company, MyoPax Denmark ApS, Spuler has been accepted into the incubator program of the BioInnovation Institute (BII) Foundation in Copenhagen, which provides financial and strategic support to help the company keep up with international competition. Meanwhile, MyoPax is preparing for expansion to the United States.
Most read news
Topics
Organizations
Other news from the department science

Get the life science industry in your inbox
By submitting this form you agree that LUMITOS AG will send you the newsletter(s) selected above by email. Your data will not be passed on to third parties. Your data will be stored and processed in accordance with our data protection regulations. LUMITOS may contact you by email for the purpose of advertising or market and opinion surveys. You can revoke your consent at any time without giving reasons to LUMITOS AG, Ernst-Augustin-Str. 2, 12489 Berlin, Germany or by e-mail at revoke@lumitos.com with effect for the future. In addition, each email contains a link to unsubscribe from the corresponding newsletter.
Most read news
More news from our other portals
Something is happening in the life science industry ...
This is what true pioneering spirit looks like: Plenty of innovative start-ups are bringing fresh ideas, lifeblood and entrepreneurial spirit to change tomorrow's world for the better. Immerse yourself in the world of these young companies and take the opportunity to get in touch with the founders.
Last viewed contents
Gene_therapy
Watching cell division live - Highest resolution insights into living cells
John_Parkinson_(botanist)

Trigger points for bowel cancer found at a young age - Regenerative stem cells as the origin of inflammation-associated intestinal tumors
List_of_Amanita_species

Energy of the future: photosynthetic hydrogen from bacteria - How cyanobacteria can be transformed into hydrogen factories
The_Doctor_(film)

Merck accelerates growth, driven by all business sectors - Merck confirms and specifies guidance for fiscal 2024

Do women age differently from men? - The biological sex may be a decisive factor in the effectiveness of anti-ageing drugs