Research team reveals hidden particle interactions at the cell surface
Ultra-smooth interactions can be detected through high-speed measurement and frequency analysis
Advertisement
Is it possible that in most measurements in the field of life sciences, important interactions remain hidden inside the cell or at the cell surface? This question has puzzled the team of laser- and bio-physicist Prof. Dr. Alexander Rohrbach from the University of Freiburg for years. He and his colleague Dr. Felix Jünger have been investigating various interactions of particles in the size range of bacteria, i.e. a few micrometres, and even of viruses, around 0.1 micrometres, on different cell surfaces. With the help of refined laser measurement technology and mathematical analysis methods, they have now succeeded in making previously hidden interactions visible. The results have been published in the journal Small. In the future, they may help to better understand how different particles bind to cells - be it viruses, bacteria, fine dust, cell debris or micelle-coated active ingredients.
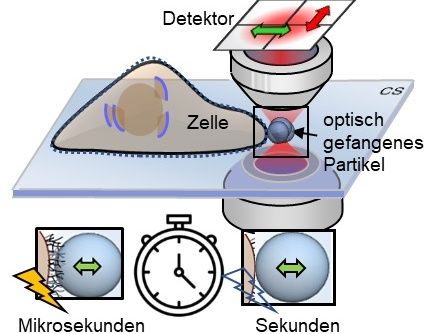
Microstructures such as the glycocalyx on the cell surface can be measured indirectly on the microsecond scale by fluctuating particles, but they remain hidden on the second scale.
Alexander Rohrbach
Viscoelasticity determines behaviour
The viscoelastic properties of cell surfaces play a decisive role here. Rohrbach cites a starch solution as an example. “If you stir a sufficient amount of corn starch into a tub of water and walk on it quickly, you can actually walk on the liquid. All you feel is an elastic surface. But if you walk slowly or stop, you sink in and feel the liquid around your feet.” So depending on the acting time scale, the solution is either elastic or viscous. The viewer sees either a person with dry or wet feet stepping out of the tub.
Biological cells consist of minute molecular structures, which are only visible under the best microscopes. They all react to pressure or tension partly elastically, i.e. energy is stored, and partly viscously, i.e. energy is lost. Each individual cell is a viscoelastic system and thus determines the viscoelastic properties, for example of muscle or connective tissue. Almost every cell has its own highly specialised extracellular matrix: a meshwork of filament-like molecules, of fine fibres and thin finger-like protrusions. This complex cell surface influences the uptake of particles such as viruses, bacteria or particulates. “During signal transduction the extracellular matrix distinguishes not only according to the size and shape and surface of the particles, but also how quickly or slowly thermally diffusing particles contact the surface, i.e., it acts as a spatial and temporal sieve for mechanical signals,” Rohrbach explains.
Extremely fast measurement technology
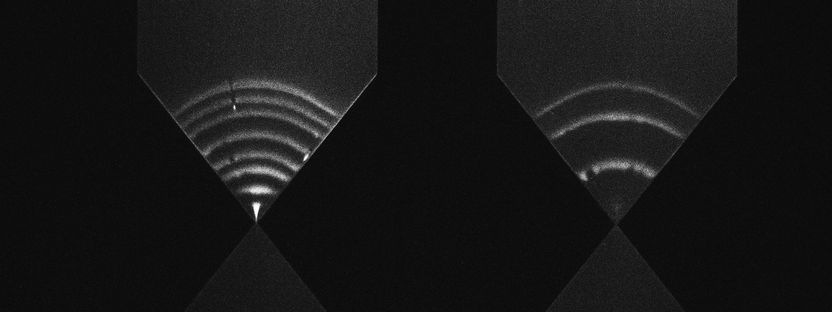
Using a so-called photonic force microscope, a combination of optical laser tweezers and an interferometric 4-quadrant detection system, bio-physicist Jünger first approached one-micrometre beads to living cells in numerous experiments. The particle, which serves as a probe, is trapped in the laser focus but still makes small tremble movements, known as thermal position fluctuations. These movements are measured three-dimensionally one million times per second - and with a precision of a few nanometres. The noisy-looking position signals of the particle, however, contain crucial information about the interaction with its environment.
If the particle is now slowly brought to the cell surface with optical tweezers or if the particle is presented to the cell at a short, constant distance, an interaction of the particle with the fine structures of the extracellular matrix begins after a few seconds. If you look at the position histogram, i.e. a distribution of all particle positions, you will notice practically no difference in the distribution before and after the interaction. The interaction is hidden. "The fast signal sampling allowed us to perform Kramers-Kronig integral transformations for thermal particle fluctuations for the first time, which allow us to visualise the elastic and viscous behaviour of cell structures at different frequencies of motion," Jünger explains. Rohrbach adds, "However, to give meaning to these two frequency responses, you have to pack your idea of what's happening on the molecular level into mathematical equations and then compare how well the solutions to these equations match the experimental results."
Mathematical minimal model in frequency domain
In doing so, the Freiburg researchers devised a mathematical minimal model in frequency domain, which can be unexpectedly well adapted to the viscoelastic behaviour in different measurement methods on different cells. By analysing the probe fluctuations, Jünger and Rohrbach were able to determine, for example, important properties of the pericellular matrix (PCM) in intestinal epithelial cells, which consists of mesh-like hyaluronic acid strands. “We were able to measure the thickness of the PCM of 350 nanometres on the microsecond scale alone; on longer time scales, the PCM was simply invisible,” Rohrbach says. The PCM’s elasticity of only about six pascals at millisecond dynamics, but of about 20 pascals at microsecond dynamics, could explain, for example, why small and highly dynamic viruses tend to bounce off the PCM more elastically from a physical point of view, while larger and less dynamic bacteria tend to sink into the PCM, precisely because it is less elastic on larger time scales.