How cells rewrite their fate
The study could be the starting point for new methods to alter the molecular mechanisms of cancer development
Advertisement
An international research team, including Achim Leutz of the Max Delbrück Center, has discovered that a specific protein controls the conversion of immune cells. If it were possible to intervene in this process, the development of cancer could be prevented.
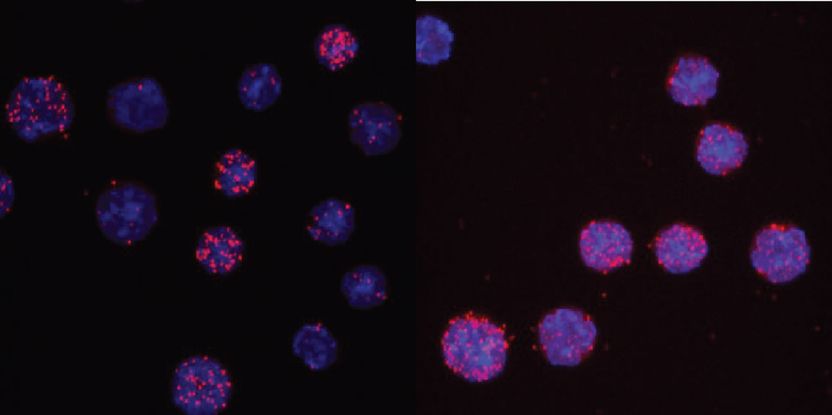
C/EBPαR35A interacts more strongly with PU.1 than unmated C/EBPα. The images show the nuclei of cells in light blue and the interaction readout as red dots. For the wild type only 3 of 13 cells show signals, while for the mutant 7 of 8 cells are positive.
Thomas Graf, Centre for Genomic Regulation
Cell fate determines which cell type a cell differentiates into and which function it assumes in the organism. If cells make the wrong decision in this process, cancer can develop.
Researchers at the Centre for Genomic Regulation (CRG) in Barcelona and the Max Delbrück Center in Berlin have investigated how how cells can accelerate their fate. They present their results in the scientific journal “eLife”. The study could be the starting point for new methods to alter the molecular mechanisms of cancer development.
Let phagocytes off the leash
Central to the study is C/EBPα (CCAAT/enhancer-binding protein alpha), a protein that orchestrates the conversion of B lymphocytes to macrophages. B lymphocytes are white blood cells that form antibodies against pathogens. Macrophages also belong to the white blood cells. Called “scavenger cells”, they find and eliminate pathogens and pathologically altered cells. C/EBPα is a transcription factor, a type of protein which binds to specific DNA sequences in the regulatory regions of genes where it activates or represses protein expression.
Like all proteins, C/EBPα consists of different amino acids. Enzymes can modify proteins by attaching various methyl groups – which are small “stickers” each consisting of one carbon atom and three hydrogen atoms – to certain amino acids. This process is called methylation. In this way, they can strongly influence protein interactions. The researchers found that when one specific amino acid residue of C/EBPα is left unmethylated, it greatly accelerates the conversion process of B lymphocytes to macrophages. The methylation of this specific amino acid residue is mediated by the enzyme Carm1. Previous research has shown that Carm1-deficient mice are resistant to induced forms of acute myeloid leukaemia. This could be due to the mechanisms the researchers revealed in their study: the unmethylated version of C/EBPα is a stronger inducer of macrophage differentiation compared to its methylated counterpart. As macrophages are a non-dividing cell type, this could prevent the formation of cancer cells.
New ideas for treating leukemia
“By understanding how cell fate conversion can be accelerated or directed, we uncover new clues for cancer research”, says senior author Dr. Thomas Graf from the CRG. “For example, targeting the balance between methylated and unmethylated forms of C/EBPα can help us understand how immune cells differentiate and eventually lead to new ideas for treating certain forms of leukaemia.”
The location of the critical amino acid within C/EBPα was found when the researchers tested a mutant form of the protein called C/EBPαR35A. This mutant dramatically accelerated the speed by which B cells could be turned into macrophages. To induce a cell conversion, C/EBPα works by interacting with another transcription factor called PU.1. C/EBPαR35A was found to interact much more strongly with PU.1 than C/EBPα. This silenced B cell genes more quickly and activated macrophage genes instead.
Drugs against “confused” cells
The methylation of C/EBPα is an example of an epigenetic mechanism. These are mechanisms which modify how the genome – the instruction manual inside every cell of the human body – is read. “Drugs that affect epigenetic mechanisms may indeed alter the function of transcription factors and correct cells that went astray, such as seen in cancer and leukaemia,” says senior author Dr. Achim Leutz, Head of the research group “Cell Differentiation and Tumorigenesis” at the Max Delbrück Center. “In this novel mechanism PU.1 is triggered by C/EBPα to switch from a B cell regulator into a macrophage regulator, an elegant ‘on-off’ mechanism that ensures the faithful formation of a mature cell type, avoiding the formation of ‘confused‘ cells often seen in blood cancers. Therefore, drugs might be found that target this mechanism to correct such defects.”
Much remains unknown about what determines the speed and directionality of cell fate decisions. The work suggests that the two processes are two sides of the same coin. For instance, how do stem cells sequentially transform into the many different types of cells in the body? Better understanding how cells change their identity and how to manipulate the process might have applications from regenerative medicine to improving the efficacy of drugs against cancer.