3D-printed pills with desired drug release – a step upwards in medication
Funny looking pills are not a design gimmick, they can release medication in a desired time regime!
Advertisement
Don’t be surprised to see pills with unusual shapes in the future. At first sight they may look funny, but they can release pharmaceuticals inside the body in a controlled manner. Using a combination of advanced computational methods and 3D printing, objects are produced that dissolve in liquids in a predetermined manner. A group of Computer Scientists from the Max Planck Institute for Informatics in Saarbrücken, Germany, and the University of California at Davis, have invented a process that relies solely on the shape of the object for a time-controlled release. This will have important implications for the pharmaceutical industry, which has recently begun focusing sharply on 3D printing.

3D printed pill
MPI-INF

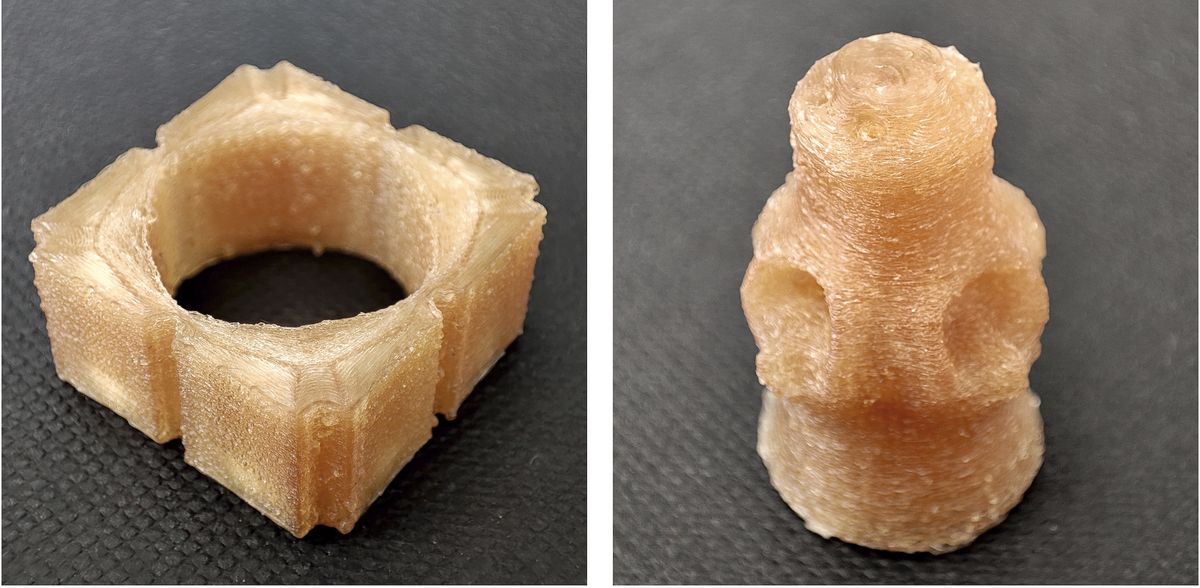
A few designs with pre-selected release regimes of medication release
MPI-INF


Controlling pharmaceutical drug levels in patients is an important part of medication. In the case of intravenous infusion, the concentration in the blood is determined by the drip rate multiplied with the proportion of the drug in the IV solution. A constant drug level might be achieved by initially administrating a large dose and maintaining it from then on by smaller doses. With oral administration, this regime is much more difficult to ensure. One idea would be to use multi-component, multi-material structures with different drug concentrations at different locations, which is difficult to manufacture. On the other hand, great advances in 3D printing and its insurmountable capabilities for creating complex shapes, making free-form drugs with a constant distribution of the biochemical in the carrier material is currently a viable option. For such drugs, the release depends solely on the geometric shape, which is easier to ensure and control.
The project, led by Dr. Vahid Babaei (MPI for Informatics) and Prof. Julian Panetta (UC Davis), produces 3D objects that dissolve in a desired function of time, hence releasing their content in a controlled way. By cleverly combining mathematical modeling, experimental setup, and 3D printing, the team can print 3D shapes that deliver a timed amount of drug as they dissolve. This can be used to set predetermined drug concentrations through oral delivery.
Since no external influence is possible after ingestion in the digestive tract, the desired time-dependent drug release must be generated by the shape (active surface that dissolves) of the specimen. With some effort, the time-dependent dissolution can be calculated from a given geometric shape. For a sphere, for example, it is strictly proportional to the decreasing spherical surface. The research team proposes a forward simulation, based on a geometric intuition that objects are dissolved one layer at a time. Practitioners are, however, mostly interested in defining a desired release first and then finding a shape that dissolves according to that release profile. Even with this efficient forward simulation, the reverse engineering to find the appropriate three-dimensional shape for a desired drug regime has significant difficulties.
This is where topology optimization (TO) finds application: forward simulations are inverted to find a shape that exhibits a certain property. Originally developed for mechanical components, TO has meanwhile gained a wide range of applications. The team is the first to propose an inverse design strategy to find the shape from release behavior based on topology optimization. The dissolution is validated by means of experiments: the measured release curves are very close to the desired values.
In the experimental setup, the objects are printed using a filament based 3D printer. The dissolution is then evaluated by a camera system, i.e., actually measured, not just calculated by a mathematical model. For this purpose, the optical transmittance of the solvent is optically recorded. In contrast to the measurement methods commonly used to date, which directly determine the active ingredient concentration (e.g. by titrating), this method is much faster and simpler to set up. Optical methods for measuring the density of active ingredients have, by the way, been in use for quite some time: when grapes are mashed for making wine, the sugar content (Öchsle) of the grape juice is determined by refractometry.
The inverse design method can also incorporate different fabricability constraints of different manufacturing systems. For example, it can be modified to generate extruded shapes and thus does not stand in the way of mass production. Beyond the discussed application in pharmaceutics, further possibilities include the production of catalytic bodies or even coarse granular fertilizers.