The folding is the key: A new Achilles’ heel in HIV discovered
Researchers at Helmholtz Institute Würzburg discover potential new antiviral targets in HIV-1
Ribonucleic acid (RNA) folds into complex structures, which allow it to interact specifically with other molecules in the cell. In HIV-1, minute differences in RNA folding can be crucial in determining whether viral RNA is “packaged” and thus leads to viral replication. This has now been discovered by researchers at the Helmholtz Institute Würzburg by enhancing a method used to study RNA structure with a novel sequencing technology. Their findings could help to design new antivirals and were published in the journal Nature Methods.

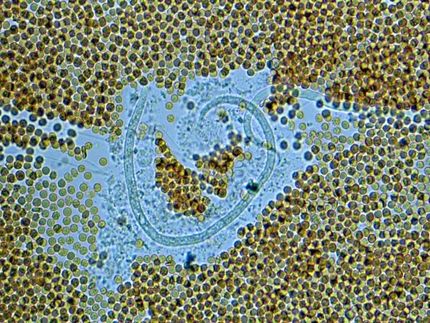
Anne-Sophie Gribling-Burrer, Redmond Smyth, Patrick Bohn, and Uddhav Ambi (left to right) have discovered a new Achilles’ heel in HIV.
HIRI/Luisa Macharowsky
Human Immunodeficiency Viruses (HIV) are responsible for millions of infections worldwide. By causing the Acquired Immunodeficiency Syndrome (AIDS), these pathogens have led to nearly 40 million deaths since the outbreak of the HIV pandemic in the 1980s. For decades, scientists have been researching possible antiviral therapeutics, and effective drugs are now available for those infected. However, it is the combination of multiple antivirals with different targets which has revolutionized HIV therapy, and new drugs are continuously needed to combat drug resistance.
“In our study, we’re introducing a possible new target to stay one step ahead of HIV and other potentially zoonotic retroviruses,” says Redmond Smyth. Smyth heads a research group at the Helmholtz Institute for RNA-based Infection Research (HIRI) in Würzburg, a site of the Braunschweig Helmholtz Centre for Infection Research (HZI) in cooperation with the Julius-Maximilians-Universität (JMU) Würzburg, and has led the current study.
“Our new method can distinguish the structural variations between highly similar RNAs, even those created through splicing,” explains Patrick Bohn, a PhD student in the Smyth lab. The researcher is the co-first author of the study, which was published in the journal Nature Methods.
Splicing is a biological process that, in a sense, refines a cell's genetic blueprint present in the original messenger RNA for subsequent translation into new proteins. “In higher organisms, splicing generates protein diversity, but our findings indicate that it can also contribute to the biological function by producing novel RNA structures,” Bohn says.
Very similar yet different
The current findings were obtained by improving a technology to measure how RNA is folded in the cell. Many scientists have attempted to study structures of spliced and unspliced HIV-1 RNA, but this has been challenging because previous technologies only measured RNA structure in small fragments. Scientists at the HIRI have now applied long read sequencing to study RNA structure across the full length of the RNA molecule, and used it to show how the HIV-1 virus selects its full-length RNA for packaging into viral particles.
“We unveiled that HIV-1 RNA folds very differently when spliced—a discovery that reveals the importance of studying biological processes taking into account the complex native environment,” says Anne-Sophie Gribling-Burrer, who is a post-doctoral researcher and co-first author on the paper. As a result, spliced versions of HIV-1 RNA were shown not to be packaged. “Spliced RNAs do not have some of the structural features that are required for being packaged, thus providing a mechanism of packaging selectivity and affecting the replication of the virus,” Gribling-Burrer explains.
“Understanding this mechanism is a key step in developing new antivirals against a wide range of retroviruses,” states Redmond Smyth. Besides that, the researchers believe their method will be useful to a broad field of molecular biologists in vastly different areas in the future.