A sweet solution to a cracking problem: Scientists design new bio-Inspired molecules to promote bone regeneration
An interdisciplinary team developed novel bio-inspired sugar-based molecules that show potential to improve bone regeneration
Advertisement
People’s ability to regenerate bones declines with age and is further decreased by diseases such as osteoporosis. To help the aging population, researchers are looking for new therapies that improve bone regeneration. Now, an interdisciplinary team of researchers from the Biotechnology Center (BIOTEC) and the Medical Faculty of TU Dresden along with a group from Max Bergmann Center of biomaterials (MBC) developed novel bio-inspired molecules that enhance bone regeneration in mice. The results were published in the journal Biomaterials.

The team: Prof. Maria Teresa Pisabarro, Dr. Gloria Ruiz Gómez, Dr. Juliane Salbach-Hirsch und Prof. Lorenz Hofbauer
TUD/Magdalena Gonciarz
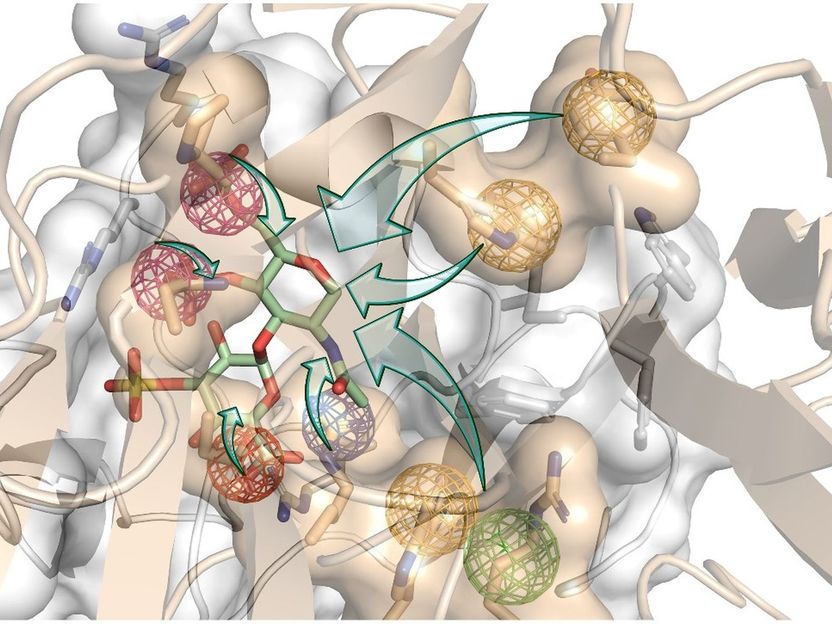
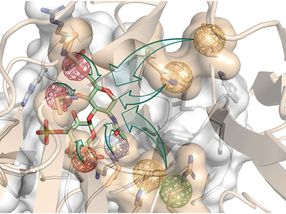
An artistic depiction of computer-aided structure-based rational design.
Gloria Ruiz Gómez

As people age, their ability to regenerate bones decreases. Fractures take longer to heal and diseases like osteoporosis only add to it. This represents a serious health challenge to the aging population and an increasing socioeconomic burden for the society. To help combat this issue, researchers are looking for new therapeutic approaches that can improve bone regeneration.
A team of scientists from Dresden used computer modeling and simulations to design novel bio-inspired molecules to enhance bone regeneration in mice. The new molecules can be incorporated into biomaterials and applied locally to bone defects. These new molecules are based on glycosaminoglycans, which are long-chained sugars such as hyaluronic acid or heparin.
A Sweet Solution for an Old Bone
“Thanks to our group’s work and the work of other researchers, we know a distinct molecular pathway that regulates bone formation and repair. In fact, we can narrow it down to two proteins that work together to block bone regeneration, sclerostin and dickkopf-1” explains Prof. Lorenz Hofbauer, “The big challenge for developing drugs that improve bone healing is to efficiently turn off both of these proteins, which act as brake signals, at the same time.”
An interdisciplinary approach was a key to this challenge. The Structural Bioinformatics group led by Prof. Maria Teresa Pisabarro at the Biotechnology Center (BIOTEC) of TU Dresden and the Functional Biomaterials group led by PD Dr. Vera Hintze at the Max Bergmann Center of Biomaterials (MBC), Institute of Materials Science of TU Dresden combined their know-how with bone expert Prof. Lorenz Hofbauer at the Medical Faculty of TU Dresden.
“For several years, we have harnessed the power of computer simulations to investigate how proteins regulating bone formation interact with their receptors. All this to design new molecules that can efficiently interfere with these interactions. We worked in tandem between the computer and the bench, designing new molecules and testing them, feeding the results back to our molecular models and learning more about the molecular properties required for our goal,” explains Prof. Pisabarro.
Finally, the team of Lorenz Hofbauer’s Bone Lab used a biomaterial loaded with the new molecules on bone defects in mice to test their effectiveness. The group found that materials containing the novel molecules outperformed the standard biomaterial and enhanced bone healing by up to 50%, which indicates their potential for improving bone regeneration.
Value-Added Chain: From Computer to the Lab Bench and Back
The multidisciplinary team used rational drug design to create novel molecules with tailored properties and minimal side effects. By using computational methods to predict and refine the properties of the designed molecules, the team was able to develop a series of candidates with the greatest potential for turning off the proteins that block bone regeneration.
Pisabarro group’s expertise allowed the thorough analysis of the three-dimensional (3D) structures of the two proteins that block bone regeneration. With that, they were able to model their interaction with their receptors in 3D and identify so-called hot spots, i.e., specific physicochemical and dynamic properties that are essential for the biological interaction to occur.
“We used molecular modeling to design new structures that mimic relevant receptor interactions with both proteins. We wanted this binding to be stronger than their natural interactions. In this way, our novel molecules would simultaneously hijack the proteins and effectively turn them off to turn the bone regeneration on,” explains Prof. Pisabarro.
“The molecules designed by Pisabarro’s group were synthesized by our colleagues at the Free University of Berlin and then analyzed regarding their protein binding properties via biophysical interaction analysis,” says PD Dr. Hintze. “For each of the molecules we were able to measure the binding strength with the proteins and their interference with natural receptor binding of the proteins. Thus, we could reveal empirically how effective each of the small molecules could be at turning off the inhibitory proteins.” Hofbauer group then tested the biological relevance of these interaction studies in a cell culture model and later in mice.
The results of such iterative testing are a valuable asset that enhances the current molecular models of the Pisabarro group and can be used to guide the development of novel and better molecules in the future. Such an approach also ensures that animal research is minimized and enters the project only in its final phase.
On the Way to Drug Development
The team's findings represent an exciting step forward in preclinical development. The newly designed molecules could potentially be used to turn off the proteins that block bone regeneration and lead to the development of novel, more effective treatments for bone fractures and other bone-related conditions.
The team continues to work together. “We are applying for funding for a pre-clinical study that will further develop the molecules and biomaterial-based bone booster to lay the ground for studies in humans,” says Prof. Hofbauer.
Original publication
Gloria Ruiz-Gómez, Juliane Salbach-Hirsch, Jan-Niklas Dürig, Linda Köhler, Kanagasabai Balamurugan, Sandra Rother, Sophie-Luise Heidig, Stephanie Moeller, Matthias Schnabelrauch, Giulia Furesi, Sophie Pählig, Pedro M. Guillem-Gloria, Christine Hofbauer, Vera Hintze, M. Teresa Pisabarro, Jörg Rademann, Lorenz C. Hofbauer: Rational engineering of glycosaminoglycan-based Dickkopf-1 scavengers to improve bone regeneration. Biomaterials (April 2023)