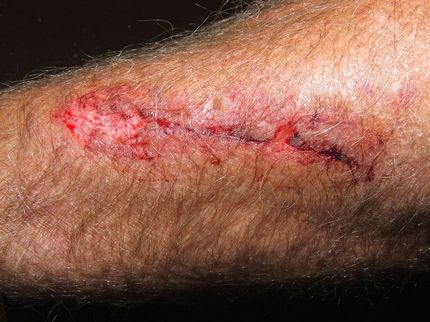
Scarless wound healing: what we can learn from spiny mice
An ERK-dependent molecular switch antagonizes fibrosis and promotes regeneration
Injury response after a deep skin wound, myocardial infarction, stroke, spinal cord injury or lung infection mostly yields fibrotic tissue, resulting in permanent scars and organ function failure. It is estimated that around 50% of people die from a disease that involves scarring. Currently, there are no treatments for restoring loss of organ function due to an injury or a pathological condition. Understanding the molecular mechanisms driving fibrosis and regeneration in adult mammals thus is of pivotal importance to develop new therapeutic strategies. In the last decade, spiny mice (Acomys species) have emerged as a robust model system to investigate tissue regeneration in adult mammals, because they can regenerate complex tissue structures after injury. A team around Kerstin Bartscherer, in collaboration with the lab of Ashley Seifert, compared cellular and molecular differences in wound healing between adult spiny mice and laboratory mice (Mus species). They identified the ERK-pathway as a molecular switch between scarless regeneration and fibrotic healing. All in all, these data suggest that a wound in adult mammals can be stimulated to regenerate without scarring by fine-tuning certain central signaling pathways.

Symbolic image
Computer-generated image
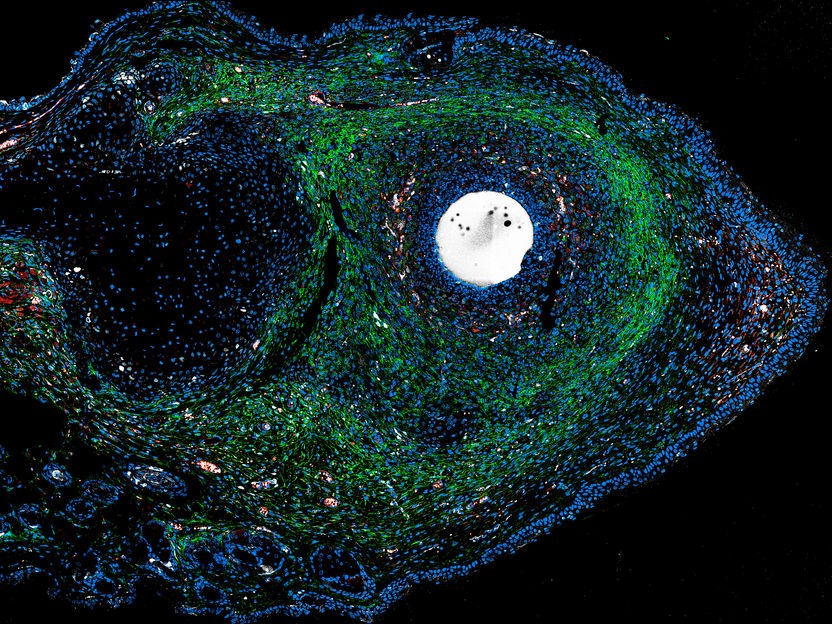
ERK activators – administered in a growth factor-impregnated microsphere – stimulate endogenous tissue regeneration in scar-prone injuries of adult mammals.
Antonio Tomasso/Max Planck Institute for Molecular Biomedicine/Hubrecht Institute

Unlike mammals and humans, some animal species are true masters of regeneration: a planarian can be cut into 200 pieces and each will grow to a complete organism again, starfish and salamanders can regrow complete arms and in zebrafish, large parts of the fin or heart can regenerate. Studies on these species have already yielded important insights into the mechanisms behind cell growth and differentiation. Yet, some questions remain or even emerge from such findings: Why do some animals regenerate better than others? What are the key molecular players of tissue regeneration? And: do mammals that normally scar upon injury have completely lost their ability to regenerate or do they still possess latent regenerative traits?
In their earlier studies, the team of Kerstin Bartscherer identified ERK pathway activation as one of the first processes in planarian regeneration. Now, the researchers aimed to find out whether this evolutionarily highly conserved signaling pathway can also trigger regeneration in mammals. To this end, they used the spiny mouse (Acomys species) as a model organism. Spiny mice have remarkable abilities of regeneration, as Ashley Seifert from the University of Kentucky (USA) discovered in 2012. For example, when ear punched, spiny mice exhibit complete regeneration of hair follicles, sebaceous glands, dermis and cartilage, hence leaving them scarless. The regenerative abilities of spiny mice even extend to other organ injuries.
“We now demonstrated that cellular ERK activity acts as a molecular switch between tissue regeneration and tissue scarring in adult mice,” Antonio Tomasso says. He is the first author of the current article in Science Advances and was a PhD student in Kerstin Bartscherer’s lab – first at the Max Planck Institute for Molecular Biomedicine in Münster (Germany), then at the Hubrecht Institute in Utrecht (The Netherlands).
Comparative studies with spiny and laboratory mice, undergoing the same type of injury, enabled the scientists to dissect the injury response in different phases. “In the lab of Ashely Seifert, I analyzed the activity of thousands of genes at once to create a global picture of cellular function, both in loss- and gain-of-function experiments,” Antonio Tomasso says.
Having identified FGF and ErbB2 receptors as of key importance to the ERK pathway in spiny mice, Antonio Tomasso could selectively inhibit pathway activity by blocking these receptors in Acomys: “ERK inhibition shifted the Acomys wound response from regeneration towards scarring, resembling the fibrotic repair observed in Mus injured tissues,” he says.
Even more intriguing were the findings after activation of the ERK pathway in normally non-regenerating Mus species: “Here, we observed a regenerative response similar to that in spiny mice,” Antonio Tomasso says. ”We even observed hair follicle regeneration – a feature that is missing in scar-building tissue repair.”
Analyses of ERK activity at a single cell level, in a collaboration with Philip Lijnzaad from the Princess Máxima Center for Pediatric Oncology in Utrecht (The Netherlands), revealed that: “During regeneration, ERK is activated in keratinocytes, being the dominant cell type constituting the epidermis and playing an essential role in skin repair, as well as in cells of the connective tissue,” Antonio Tamasso says. “In fact, these data suggest that ERK activity has the capacity to rewire connective tissue cells towards an otherwise latent regenerative program.”
“Interestingly, we observed sustained high ERK activation not only during ear tissue regeneration, but also during recovery of skin and after myocardial infarction in Acomys,” Kerstin Bartscherer says. “This indicates that sustained high ERK activity might be a general feature of regenerating tissues.”
“In order to assess the functional role of ERK activity in other injury paradigms that form a scar, such as myocardial infarction, lung infection or spinal cord injury, we need additional studies,” Kerstin Bartscherer says. “It would be interesting to test whether e.g. small molecules can safely activate ERK activity locally when administered in the injured organ in order to promote regeneration.” All animal experimental work was performed at either the University of Kentucky or the Hubrecht Institute according to strict guidelines. The work was supported by a grant from the European Research Council (ERC-StG-IniReg) to Kerstin Bartscherer.