A new, sustainable source for a promising cancer killer
Rare, mysterious plant chemical to potential anticancer drug candidate
Plants produce all types of curious chemicals. Some deter predators. Some smell wonderful. Some even have medicinal value. One of these hidden gems is (–)-jerantinine A (JA), a molecule with remarkable anticancer properties, produced by a plant called Tabernaemontana corymbosa. Unfortunately, access to this Malaysian jungle plant and its promising chemical compound has been limited. Until now.
Symbolic image
Computer-generated image
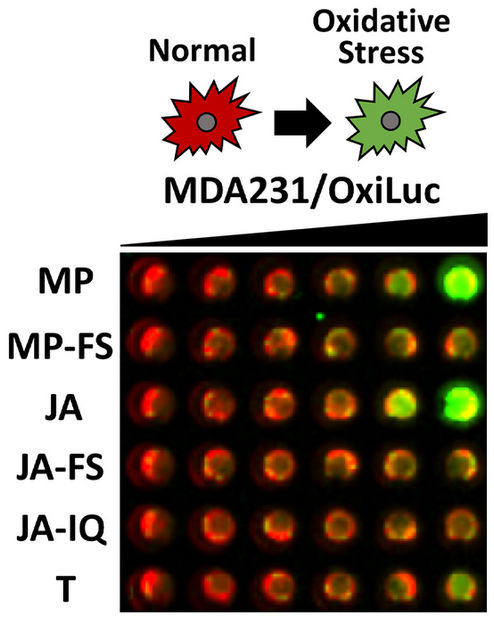
A new firefly light-based assay, called OxiLuc, developed by CSHL Research Associate Professor and Director of Animal Imaging Scott Lyons, captures oxidative stress on cells in real time. In this image, green represents the level of stress a cell is under—the greener the image, the higher the cellular stress. CSHL scientists discovered (–)-jerantinine A (row labeled JA) causes human triple-negative breast cancer cells to suffer distress from elevated oxidation levels.
©Cold Spring Harbor Laboratory
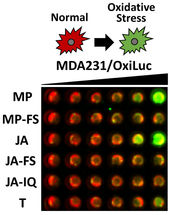
Cold Spring Harbor Laboratory (CSHL) chemists, led by Professor John E. Moses, have created a way to safely, quickly, and sustainably synthesize JA in the lab. To cancer biologists at CSHL, this breakthrough could mean future treatments for triple-negative breast cancer.
JA is attractive because of its versatility. Described as a polypharmaceutical, the molecule has the power to attack cancer from multiple angles. This can help its efficacy and reduce the chances of drug resistance. “That’s why natural products are often good starting points for anticancer drugs,” says Joshua Homer, a postdoc in Moses’ lab. “JA is a sophisticated molecule. Evolution has fine-tuned it for us.”
“Nature provided the blueprint,” Moses adds. “Nature provides many lifesaving molecules, but it doesn’t always provide them in large quantities. But we can now access (–)-Jerantinine A from a commercially viable source, which is very inexpensive, and make this precious material.”
JA also has a peculiar preference for killing cancer cells over normal cells. To find out why, the researchers enlisted a CSHL Cancer Center colleague, Assistant Professor Michael Lukey. The team discovered that JA shuts down metabolic activity in the mitochondria of human triple-negative breast cancer cells. By inhibiting mitochondria, the cells’ “powerhouse,” the molecule starves tumors of the excessive energy and building blocks they typically demand.
“The metabolic requirements of the cancer cell are very different from those of the healthy cell,” Lukey explains. “There’s now an additional requirement to make lipids, proteins, etc., for building a new cell. If the cell doesn’t have the materials needed to proliferate, it can lead to a metabolic crisis that ultimately results in cell death.”
Easy access to this new molecule means a new generation of drugs may be on the horizon for breast cancer and potentially other cancers as well. Zifei Wang in the Moses lab is now spearheading this effort. Their team sees JA’s journey from rare, mysterious plant chemical to potential anticancer drug candidate as a “poster child” of what can happen when chemistry and biology labs come together at CSHL.
“This represents the first collaborative publication by the Moses laboratory, a world-class chemistry group, working with CSHL cancer biologists to investigate novel compounds for potential anticancer properties,” says CSHL Cancer Center Director David Tuveson. “It is exciting to see this synergy at CSHL and emblematic of success stories to come in 2023!”